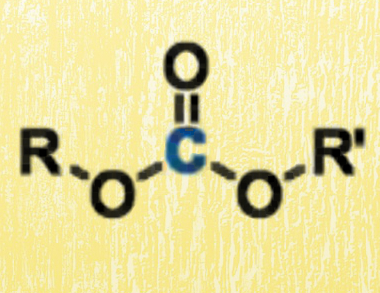
Organic carbonates (pictured) are an important class of compounds that are easily available from CO or CO2, and their selective reduction has attracted much interest. Dimethyl carbonate, in particular, has found application as a green methylating reagent.
Matthias Beller and colleagues, Leibniz-Institut für Katalyse, Rostock, Germany, have developed a reductive methylation of amines using organic carbonates to afford N-methyl amines, a key intermediate for organic synthesis. Established routes to N-methyl amines use CO2 or formaldehyde as methylating agents, which require the use of high pressure. In contrast, the use of organic carbonates allows N-methylation of amines under ambient conditions.
Key to the reaction is a catalyst formed in situ from Karstedt’s complex ([Pt(CH2=CHSiMe2)2O]) and 2,2′:6′,2”-terpyridine. With this catalyst system, the researchers were able to perform reductive methylations of a wide range of secondary amines, as well as formamide (an identified reaction intermediate), in up to 94 % yield. The catalyst was also applicable to the selective monomethylation of heterocyclic diamines. In the absence of amines, the system allowed the room-temperature reduction of carbonates to methanol.
- Convenient Reductive Methylation of Amines with Carbonates at Room Temperature,
Yuehui Li, Iván Sorribes, Cristian Vicent, Kathrin Junge, Matthias Beller,
Chem Eur. J. 2015.
DOI: 10.1002/chem.201502917