For chemical and petrochemical industries, separation of saturated and unsaturated hydrocarbons, such as alkens and paraffins, is important. Petroleum refining and chemicals manufacturing are among the most energy-intensive industries. Development of new cost-effective technologies aims at reducing the high energy consumption during the separation process.
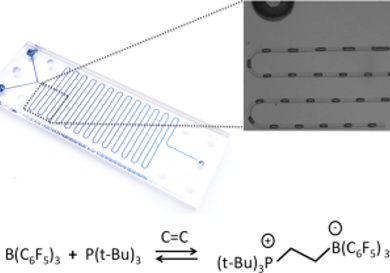
Dan Voicu, Douglas W. Stephan, and Eugenia Kumacheva, University of Toronto, ON, Canada, investigated the separation of ethylene and ethane. They used the reaction of ethylene with frustrated Lewis pair (FLP) reagents, namely, tri-tert-butylphosphine, (P(tBu)3), and tris(pentafluorophenyl) borane, B(C6F5)3, and studied the separation process using segmented gas–liquid microfluidic (MF) flow. This enabled a rapid, high-throughput assessment of reaction conditions to optimize gas separation efficiency.
Higher FLP concentration and lower temperature of the system shifts the chemical equilibrium of the reaction of ethylene with FLP towards the product. The team achieved efficient separation with a separation factor of 7.3 for ethylene from a 1:1 volume ratio mixture of ethylene and ethane and 88 % ethylene purity. The results were validated using infrared spectroscopy.
The researchers say that the results of their work provide pave the way for future exploratory work on FLP applications in the separation of gases. Their future work will focus on thermodynamic characterization of the reaction of ethylene with FLPs, investigation of the reversibility of the reaction at elevated temperature, and on regeneration of the FLP upon efflux of the desired gas.
- Microfluidic Separation of Ethylene and Ethane Using Frustrated Lewis Pairs,
Dan Voicu, Douglas W. Stephan, Eugenia Kumacheva,
ChemSusChem 2015.
DOI: 10.1002/cssc.201501160