Microalgae, as one of the most important global biomass producers, do not only largely contribute to our global oxygen production, they are also able to produce several high value compounds with a high nutritional value. Next to natural antioxidant colorants, microalgae can contain high amounts of protein and omega-3-fatty acids which may help to prevent heart diseases. On the other hand, microalgae produce oil which might be a future fuel. Nevertheless, there is still a lot of research to do to make these things profitable.
1 Life at the Beginning of the Food Chain
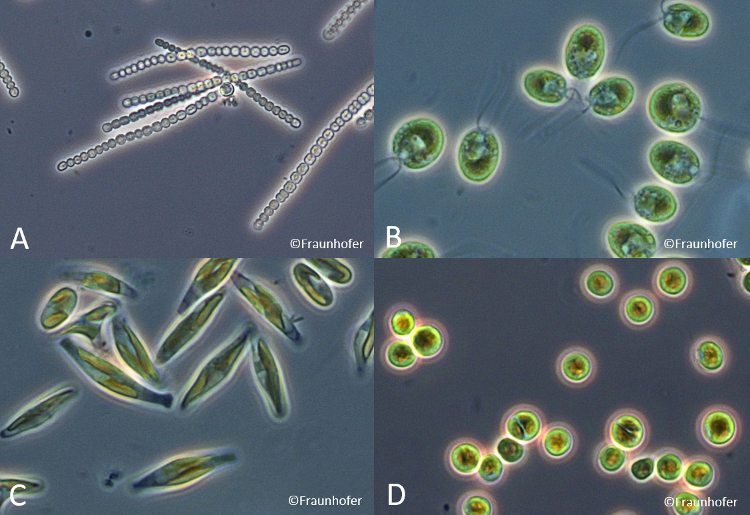
Microalgae represent a diverse group of plant-like, unicellular organisms. Nowadays, it is estimated that about 300.000 different species exist on earth. Around 40.000 species are actually described and a few are analyzed in detail [1,2]. The term “microalgae” includes prokaryotic cyanobacteria as well as eukaryotic microalgae species (Fig. 1) capable of growing in presence of sea water (e.g. oceans), fresh water (e.g. lakes, rivers) and on several kinds of ground surfaces (e.g. soil) [3].
|
|
|
Figure 1. Microscopic photographies (magnification 1:1000) of different microalgae species. A: Anabaena, B: Chlamydomonas reinhardtii, C: Phaeodactylum tricornutum, D: Chlorella sorokiniana. Anabaena is a cyanobacterium, Chlamydomonas reinhardtii and Chlorella sorokiniana are green algae, Phaeodactylum tricornutum is a diatom. |
Depending on the species, microalgae are able to grow heterotrophically, mixotrophically, or under photoautotrophic conditions [4–6]. When cultivated under photoautotrophic conditions, microalgae capture light and use its energy to convert carbon dioxide – one of the most abundant greenhouse gases – via photosynthesis into chemical energy in form of carbon rich biomass. Like terrestrial plants, microalgae use nitrogen and phosphorus as fertilizer for optimal growth. By converting CO2 into organic biomass, microalgae largely contribute to the global oxygen production. It is estimated that about 50 % of the global oxygen is produced by microalgae.
2 Advantages Compared to Terrestrial Plants
Compared to higher plants, the cultivation of microalgae has a considerable number of advantages [7]. These include a five to ten times higher biomass productivity compared to terrestrial plants, along with the possibility to cultivate microalgae in controlled reactor systems on land which is not suitable for conventional agricultural purposes. Closed reactor systems lead to a considerable reduced water consumption compared to land plants since no water is lost due to evaporation or infiltration (see Figs. 2 and 4). Since several microalgae species can be cultivated in brackish or coastal seawater, the consumption of fresh water is additionally reduced.
The cultivation in reactor systems offers the possibility to influence the ingredients of microalgae biomass by regulating different process parameters, especially nutrient supply and light intensity [8]. Carbon dioxide, nitrogen, and phosphorus can be supplied using flue gas from power plants or nutrient rich waste water streams [9].
3 Reactor Systems for Algae Production
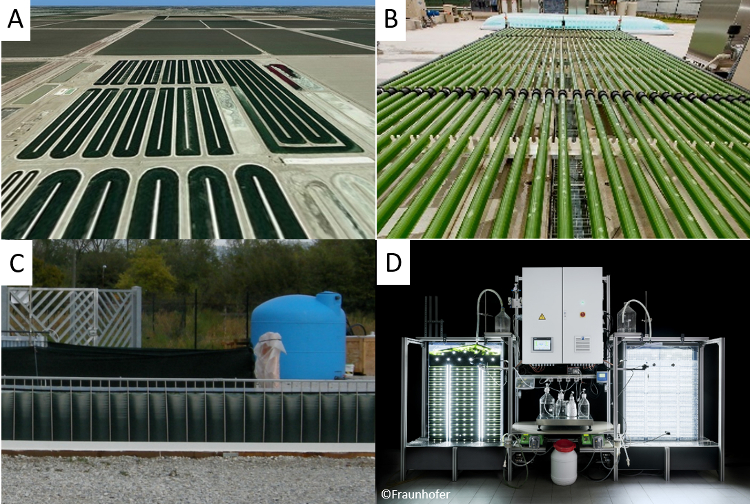
One major challenge when cultivating phototrophic organisms is to provide sufficient light for the culture. Thus many different systems for algae cultivation have been developed in the last 60 years. There are open systems and closed systems for algae cultivation. The system used is determined by the desired product and the algae species, as all systems have certain advantages and disadvantages [10]. The most common systems are open ponds, tubular reactors, and flat-panel-reactors (see Fig. 2).
|
|
|
Figure 2. Different reactor systems for algae cultivation. A: open pond in southern California [11], B: tubular reactor in the AlgaePARC in Wageningen [12], C: flat panel reactor (Greenwall panel) developed at the University of Florence [13], D: flat panel airlift reactor with control system at Fraunhofer IGB. |
3.1 Open Ponds
Open ponds are natural or artificial lakes with a culture depth of about 20 to 30 cm. These reactors reflect mostly the natural algae environment. The first open ponds were built in the 1950s and research in open ponds continues until today [9]. Raceway ponds (see Fig. 2 (A)) are an improvement of the simple ponds usually mixed with a paddle wheel to generate a higher flow velocity. Cultivating algae in open ponds is an established technology with low investment costs. Furthermore, ponds are simple to operate.
Disadvantages of this technology are low process control (e.g. lack of cooling, difficult CO2 supply, high water evaporation rate) and the low biomass productivity, as well as the low concentration (approx.1 g L–1) which can be achieved because of insufficient light supply as a result of inadequate mixing conditions. The open system also leads to a high contamination risk with other algae, bacteria, or predators. Especially extremophile algae like Spirulina, which tolerates a high pH value, or Dunaliella salina, which is able to sustain high salt concentrations, are cultivated commercially in open ponds.
3.2 Tubular Reactors
In tubular reactors, the biomass is pumped through transparent tubes with a diameter of several centimetres and a length of up to 100 m. CO2 is usually supplied in a closed mixing tank next to the tubes, where no photosynthesis can take place. The CO2 consumption and oxygen production of the microalgae can lead to pH and oxygen gradients in the culture as the flow in the tubes is usually non-turbulent.
The closed system enables the production of microalgae which cannot be cultivated in open ponds due to the low process control and the high contamination risk which can be harmful for the algae and inadequate for food safety reasons. Examples for microalgae grown in tubular reactors in large scale are Chlorella vulgaris for food supplements or Haematococcus pluvialis for the production of astaxanthin, a red colorant [14].
3.3 Flat Panel Reactors
As light is the most important parameter in algae cultivation, reactors with a high surface-to-volume ratio were developed. Flat panel reactors are, in contrast to the horizontal shape of an open pond, vertical systems with only a few centimeters culture depth and are mixed by bubbling gas at the bottom (see Fig. 4). Due to the high gas flow, no oxygen accumulation occurs and the high light availability leads to high productivities and a high biomass concentration. This concentration can be more than ten times higher than in open ponds.
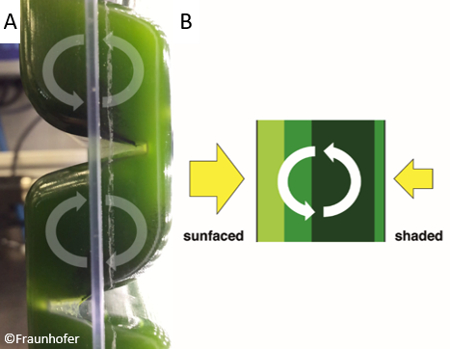
Nevertheless, in conventional flat panel reactors there is only low horizontal mixing as the gas bubbles only move straight upwards. For this reason, the flat panel airlift (FPA) reactor was developed at the Fraunhofer Institute for Interfacial Engineering and Biotechnology. It has static mixers leading to a circular current in each chamber of the reactor [15]. The flow pattern brings the algae constantly from the dark side to the light side of the reactor (Fig. 3). This optimal light distribution results in very high productivities (up to 2 g L–1 d–1) and leads to a high biomass concentration of up to 20 g L–1.
|
|
|
Figure 3. Side view of the flat panel airlift reactor showing the circular current within the compartments. |
The automation system allows full control of CO2, temperature, pH value, and nutrient concentration in the culture [8]. The reactors can be used indoors, illuminated with LEDs, and outdoors, operating with natural sunlight (Fig. 4). This reactor, combined with a high degree of automation, can be capable of net energy production with algae.
|
|
|
Figure 4. Outdoor research plant with flat panel airlift reactors at Fraunhofer IGB, Stuttgart, Germany. |
4 Ingredients and Products Derived from Microalgae
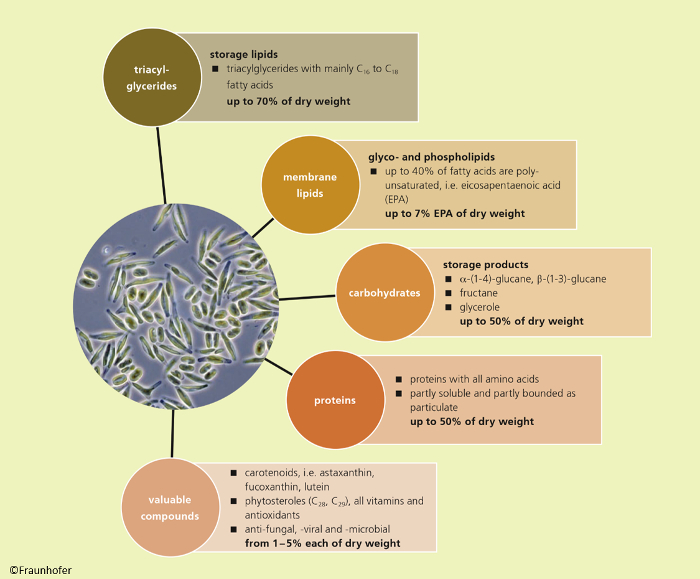
Microalgae biomass contains a huge variety of ingredients (see Fig. 5). Depending on the selected strain and the conditions (i.e., nutrient supply, light intensity, pH value, and temperature) used for cultivation, the composition of a microalgae species can vary a lot [16,17]. When cultivated with sufficient nutrient supply (nitrogen, phosphorus), microalgae contain high amounts of proteins. These proteins can constitute up to 60 % of the total dry cell mass and seem to be very suitable for food and feed purposes, since the amino acid profile is well balanced compared to the recommendations by the WHO/FAO [18,19]. A commercial product is offered by Roquette in Klötze who sell Chlorella tablets as a dietary supplement. Since Chlorella is rich in chlorophyll and lutein, the product is suspected to constitute a health beneficial supplement concerning oxidative stress and several diseases like age-related macular degeneration (ARMD) [Quelle].
|
|
|
Figure 5. Possible ingredients of microalgae biomass. |
In addition, some microalgae species, for example Phaeodactylum tricornutum, Pavlova lutheri, or Nannochloropsis oceanica, consist of phospho- and galactolipids with polyunsaturated omega-3 fatty acids, especially eicosapentaenoic acid (EPA, C20:5, ω3), and docosahexaenoic acid (DHA, C22:6, ω3) as a constituent of the chloroplast membrane [21–23]. In human nutrition, EPA can act as a precursor of prostaglandin-3 and is a typically constituent of fish oil. Prostaglandin-3 can inhibit platelet aggregation. It is also suspected that a specific EPA intake can lower inflammation and lower depression symptoms [24].
When microalgae cells are cultivated under nitrogen or phosphor starvation, some species, for example Chlorella vulgaris, are able to accumulate huge amounts of triacylglycerids consisting of glycerol and saturated lipids (mainly C16-C18 fatty acids). Under appropriate conditions, these fatty acids constitute more than 60 % of the total dry mass [8]. Other species like Chlorella sorokiniana are able to accumulate large amounts of carbohydrates as starch. Extraction of carbohydrates and unsaturated fatty acids is simple. These products are of interest for the energy sector, since they can be converted to bioethanol or biodiesel, respectively, or used as platform chemicals for further synthesis [25]. However, until now all studies and estimations confirmed that the production of biodiesel from microalgae is still too expensive and not yet competitive with fossil fuels [26, 27].
In addition to the main products mentioned above, microalgae biomass can include several high value by-products like carotenoids (e.g., astaxanthin, β-carotene, fucoxanthin, and lutein) and phytosterols, which are of interest because of their antioxidant and anti-inflammatory properties [28–31]. Some carotenoids can be used as natural and healthy food colorants. Several companies focus on this field of application, for example, Algatech in Israel, who produce astaxanthin with Haematococcus pluvialis in closed reactors.
5 Outlook
Although there are several companies producing microalgae biomass as well as different microalgae based products, there is still a huge demand for further research. At the present time only Spirulina and Chlorella are approved for direct food use. Dunaliella is approved for the production of β-carotene and Haematococcus for astaxanthin [32].
Due to the high diversity of microalgae species, there is still a big lack of information concerning growth conditions, metabolic products, extraction procedures, and food safety. There can be fundamental differences in the optimal growth conditions for different species, which hinders the establishment and approval of new species in the food and feed market. The holistic utilization of biomass is investigated by some research programs, for example the “Bioeconomy Research Program” funded by the Ministry of Science, Research and Art, Baden-Württemberg, Germany. Consumer acceptance for algae products as a vegan protein source is improving, but further marketing is necessary to establish algae as food or food ingredient.
Microalgae are also promising in cleaning waste streams, especially from nitrate, ammonium, and phosphorus. Additionally, between 1.6 and 2 kilograms of CO2 are captured per kilogram algae biomass. Thus, algae have a big potential of recycling inorganic liquid or gaseous waste streams back to organic biomass in a more efficient way than land plants. Nevertheless, the use of this biomass is difficult due to food safety reasons.
The future of algae lies especially in high value products and the biorefinery approach to generate more than one product based on one specific algae.
Authors
Felix Derwenskus and Claudia Holdmann
Universität Stuttgart, Institut für Grenzflächenverfahrenstechnik und Plasmatechnologie IGVP
Grenzflächenverfahrenstechnische Prozesse
c/o Fraunhofer-Institut für Grenzflächen- und Bioverfahrenstechnik IGB
Abteilung Umweltbiotechnologie und Bioverfahrenstechnik
Nobelstraße 12, 70569 Stuttgart, Germany
6 References
[1] A. P. Batista et al., Algal Res. 2013, 2, 164–173. DOI: 10.1016/j.algal.2013.01.004
[2] T. M. Mata, et al., Renewable Sustainable Energy Rev. 2010, 14, 217–232. DOI: 10.1016/j.rser.2009.07.020
[3] Amos Richmond (ed.), Handbook of Microalgal Culture: Biotechnology and Applied Phycology, Wiley-Blackwell, Oxford, UK, 2004. DOI: 10.1002/9780470995280
[4] D. Morales-Sánchez et al., Algal Res. 2014, 5, 61–69. DOI: 10.1016/j.algal.2014.05.006
[5] O. Perez-Garcia et al., Water Res. 2011, 45, 11–36. DOI: 10.1016/j.watres.2010.08.037
[6] M.C. Cerón-García et al., Bioresour. Technol. 2013, 147, 569–576. DOI: 10.1016/j.biortech.2013.08.092
[7] U. Schmid-Staiger et al., Chem. Ing. Tech. 2009, 81, 1783–1789 (in German). DOI: 10.1002/cite.200900079
[8] R. Münkel et al., Biotechnol. Bioeng. 2013, 110, 2882–2893. DOI: 10.1002/bit.24948
[9] P. Das et al., Bioresour. Technol. 2015, 192, 97–104. DOI: 10.1016/j.biortech.2015.05.019
[10] R.N. Singh et al., Renewable Sustainable Energy Rev. 2012, 16, 2347–2353. DOI: 10.1016/j.rser.2012.01.026
[11] F. White, PNNL Studies Algae Petroleum Replacement, AlgaeIndustryMagazine.com, April 14, 2011.
[12] Wageningen UR, Cost-effective algae production within reach, wageningenur.nl, November 18, 2014.
[13] M. R. Tredici, Energy balance of microalgae cultures in photobioreactors and ponds, EU Workshop: Life Cycle Analysis of Algal Based Biofuels, 2012.
[14] O. Pulz, Appl. Microbiol. Biotechnol. 2001, 57, 287–293. DOI: 10.1007/s002530100702
[15] P. Bergmann et al., Chem. Ing. Tech. 2013, 85, 202–205. DOI: 10.1002/cite.201200132
[16] D. Pal et al., Appl. Microbiol. Biotechnol. 2011, 90, 1429–1441. DOI: 10.1007/s00253-011-3170-1
[17] K. J. M. Mulders et al., Algal Res. 2014, 6, 8–16. DOI: 10.1016/j.algal.2014.08.006
[19] A.-V. Ursu et al., Bioresour. Technol. 2014, 157, 134–139. DOI: 10.1016/j.biortech.2014.01.071
[20] Richer S, Stiles W, Statkute L, et al., Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial), Optometry 2004, 75 (4), 216–30. DOI: 10.1016/s1529-1839(04)70049-4
[21] G. C. Zittelli et al., J. Biotechnol. 1999, 70, 299–312. DOI: 10.1016/s0168-1656(99)00082-6
[22] L. Krienitz et al., Limnologica 2006, 36, 204–210. DOI: 10.1016/j.limno.2006.05.002
[23] S. Pieber et al., Biomass Bioenergy 2012, 47, 474–482. DOI: 10.1016/j.biombioe.2012.10.019
[24] J. G. Martins, EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials, J. Am. College Nutr. 2009, 28 (5), 525–42. DOI: 10.1080/07315724.2009.10719785
[25] R. Harun, Renewable Sustainable Energy Rev. 2010, 14, 1037–1047. DOI: 10.1016/j.rser.2009.11.004
[26] L. Rodolfi et al., Biotechnol. Bioeng. 2009, 102, 100–112. DOI: 10.1002/bit.22033
[27] N.-H. Norsker et al., Biotechnol. Adv. 2011, 29, 24–27. DOI: 10.1016/j.biotechadv.2010.08.005
[28] F. Ahmed et al., Food Chem. 2014, 165, 300–306. DOI: 10.1016/j.foodchem.2014.05.107
[29] M. D. Macías-Sánchez et al., J. Supercrit. Fluids 2007, 39, 323–329. DOI: 10.1016/j.supflu.2006.03.008
[30] F. Ahmed et al., Algal Res. 2015, 10, 210–217. DOI: 10.1016/j.algal.2015.05.013
[31] M. Francavilla et al., Bioresour. Technol. 2010, 101, 4144–4150. DOI: 10.1016/j.biortech.2009.12.139
[32] P. Spolaore et al., J. Biosci. Bioeng. 2006, 101, 87–96. DOI: 10.1263/jbb.101.87