The interplay between the redox properties of substrates and metal catalysts may lead to drastically different results. As an example, the enantioselective alkylation of oxindole derivatives with chiral copper(II) catalysts produces racemic mixtures, whereas high enantioselectivity is observed when zinc(II) catalysts are used. This behavior contrasts with the previous observation that the structurally related cyclic β-ketoesters are converted with high selectivity when using the copper catalysts.
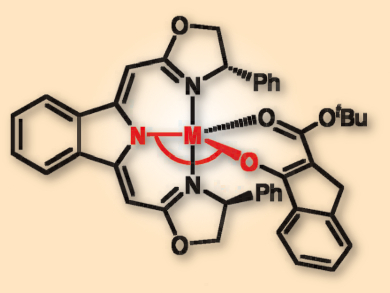
Lutz H. Gade, University of Heidelberg, Germany, and colleagues have used kinetic analysis, X-ray crystallography, NMR spectroscopy, and density functional theory (DFT) calculations to study these reactions. The team could show that the stereochemical rigidity of the coordination sphere in copper(II) catalysts is the key to their enantioselectivity in the electrophilic alkylations of β-ketoesters (pictured). However, this course of reaction is outweighed by a radical process for the catalytic transformation of oxindoles, which gives rise to racemic products.
Understanding the mechanistic basis for this unexpected behavior led the researchers to the development of a synthetically useful method involving zinc(II) Lewis acid catalysts for the alkylation of 3-monosubstituted oxindoles. The insight obtained may aid a more rational catalyst design for these types of transformations in the future.
- Radical Changes in Lewis Acid Catalysis: Matching Metal and Substrate,
Tim Bleith, Qing-Hai Deng, Hubert Wadepohl, Lutz H. Gade,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201603072