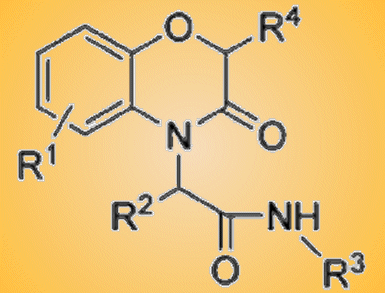
A tandem Ugi/Mitsunobu protocol for the generation of benzo[b][1,4]oxazin-3-ones with the introduction of up to four diversity inputs has been described by a team led by Luca Banfi, University of Genova, Italy.
The Ugi multicomponent reaction (MCR) is well suited for diversity-oriented syntheses, as it can introduce four diversity points in just one step. Moreover, the Ugi MCR dramatically increases structural complexity and is also “atom-economical”, as the only side product is water. If an alcohol moiety and a suitable nucleophile (phenol, sulfonamide) are introduced into two of the four components of the Ugi MCR, a post-condensation Mitsunobu cyclization is possible, which can lead to a variety of oxaza or diaza heterocycles. The combination of the Ugi and Mitsunobu reactions can provide important benzo[b][1,4]oxazin-3-one derivatives, which are important in drug design.
The mildness of the methodology allows the stereospecific synthesis of enantiomerically pure products as well as the introduction of additional functional groups. The overall two-step procedure can also be carried out in a one-pot manner. Thus, this method can be applied in the combinatorial synthesis of complex conformationally biased peptidomimetics.
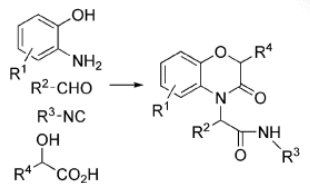
- Tandem Ugi MCR/Mitsunobu Cyclization as a Short, Protecting-Group-Free Route to Benzoxazinones with Four Diversity Points
L. Banfi, A. Basso, L. Giardini, R. Riva, V. Rocca, G. Guanti,
Eur. J. Org. Chem. 2011, 1, 100–109.
DOI: 10.1002/ejoc.201001077