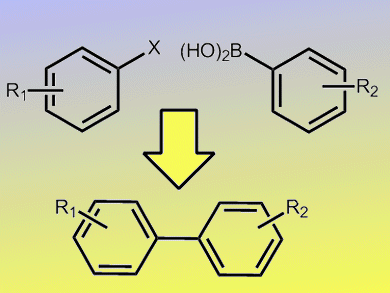
Transition-metal-catalyzed Suzuki–Miyaura reactions are an important and powerful tool for the construction of biaryl motifs commonly found in natural products, pharmaceuticals, and chemical materials. Although palladium catalysts have dominated these transformations over the past decades, efforts to explore other metal alternatives have been attracting considerable interest.
In this regard, the nickel-catalyzed Suzuki–Miyaura reactions perform excellently. The advantages of catalyst systems with nickel over those with palladium are mainly their low cost and high reactivity toward challenging substrates (e.g., deactivated aryl chlorides and sulfonates) without the need of special ligands.
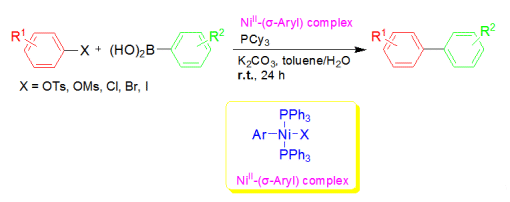
Room-temperature transformations also represent a highly desirable process from a synthetic point of view. Xin-Heng Fan and Lian-Ming Yang, Chinese Academy of Sciences, have reported the room-temperature Suzuki–Miyaura aryl–aryl cross-coupling. This was achieved by using an easily-accessible, air-stable NiII–(σ-aryl) complex as precatalyst without the aid of any organometallic reagent or external reductant. The NiII complex, in conjunction with some phosphane ligands, was able to efficiently cross-couple aryl sulfonates (OTs, OMs) and/or halides (Cl, Br, I) with arylboronic acids at room temperature in toluene/water in the presence of K2CO3 as base.
This facile, mild, and general protocol represents a new advance in Suzuki cross-coupling reactions.
Images: (c) Wiley-VCH
- Room-Temperature Nickel-Catalysed Suzuki–Miyaura Reactions of Aryl Sulfonates/Halides with Arylboronic Acids
X.-H. Fan, L.-M. Yang,
Eur. J. Org. Chem. 2011.
DOI: 10.1002/ejoc.201001519