Polyhydroxyalkanoates (PHAs) are natural polyesters produced by microorganisms as distinct granules. They are used as energy storage materials. PHAs have attracted intense attention as substitutes for petroleum-based plastics and as elastomers in applications such as packaging and medicine. PHA synthase (PhaC) is the key enzyme responsible for PHA biosynthesis. It shows broad substrate specifity. However, it’s structure and mechanism have long been unclear.
Sang Yup Lee, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Kyung-Jin Kim, Kyungpook National University, Daegu, both Republic of Korea, and colleagues have determined the structure of PhaC and have described the molecular mechanisms of the PHA biosynthesis.
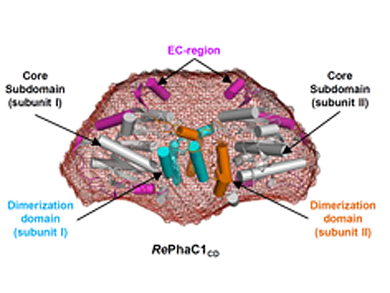
The researchers reported the crystal structure of the C-terminal domain of PHA synthase from Ralstonia eutropha (RePhaC1). They reported the first 3D reconstructed model of the full R.eutropha PhaC1 (RePhaC1F) by small angle X-ray scattering (SAXS) analysis.
RePhaC1 is a dimer of two distinct domains, the N-terminal domain (RePhaC1ND) and the C-terminal domain (RePhaC1CD). RePhaC1CD is located at the center of RePhaC1F. RePhaC1ND is located opposite of RePhaC1CD. RePhaC1CD catalyzes polymerization via a non-processive ping-pong mechanism using a Cys-His-Asp catalytic triad. RePhaC1ND is important for PHA polymerization by localizing the enzyme to the PHA granules and stabilizing the growing PHA polymer near the active site of RePhaC1CD.
According to the researchers, their findings have high potential for tailor-made PHAs that could potentially replace petroleum-based products.
- Crystal structure of Ralstonia eutropha polyhydroxyalkanoate synthase C-terminal domain and reaction mechanisms,
Jieun Kim, Yeo-Jin Kim, So Young Choi, Sang Yup Lee and Kyung-Jin Kim,
Biotechnol. J. 2016.
DOI: 10.1002/biot.201600648 - Structure and function of the N-terminal domain of Ralstonia eutropha polyhydroxyalkanoate synthase, and the proposed structure and mechanisms of the whole enzyme,
Yeo-Jin Kim, So Young Choi, Jieun Kim, Kyeong Sik Jin, Sang Yup Lee, Kyung-Jin Kim,
Biotechnol. J. 2016.
DOI: 10.1002/biot.201600649