Cyclopropanes are useful synthetic intermediates and common groups in pharmaceutically active compounds. One approach for their enantioselective synthesis is the organocatalyzed reaction of bromomalonates with α,β-unsaturated aldehydes. The reaction can be catalyzed by silylated diarylprolinols. However, there are side reactions between the cyclopropane and the base used in this process that lead to unwanted byproducts. Reducing the contact time between the catalyst and base by using flow- instead of batch processes could solve this problem.
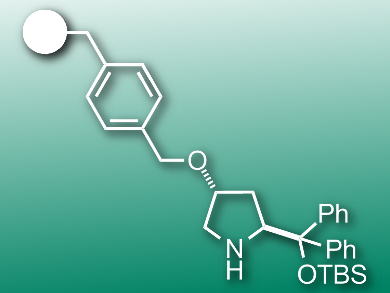
Carles Rodríguez-Escrich, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, Miquel A. Pericas̀, ICIQ and Universitat de Barcelona, Spain, and colleagues have developed a continuous-flow process which uses an immobilized diarylprolinol catalyst for cyclopropanation. The team attached the catalyst to polystyrene using a benzyl linker (pictured). They packed the resulting resin into a column built into a flow setup. Solutions of the reagents and the base N-methylimidazole were pumped through the column at rates of 50 μL/min. The base was removed behind the column with an aqueous ammonium chloride solution.
The catalytic resin retained its activity in the flow process for over 48 hours. The researchers prepared 12 different substituted cyclopropanes using this approach and achieved excellent diastereoselectivities and enantioselectivities. The amount of byproducts was reduced compared to a similar batch process.
- Organocatalytic Enantioselective Continuous-Flow Cyclopropanation,
Patricia Llanes, Carles Rodríguez-Escrich, Sonia Sayalero, Miquel A. Pericàs,
Org. Lett. 2016.
DOI: 10.1021/acs.orglett.6b03156