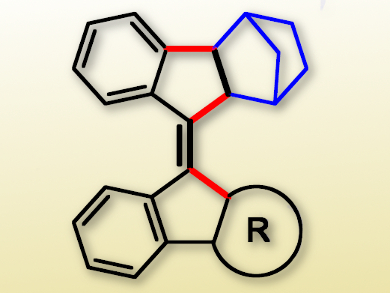
The selective synthesis of tetrasubstituted olefins is still a challenge in organic synthesis. Paramasivan Thirumalai Perumala, CSIR-Central Leather Research Institute, Chennai, India, and colleagues have developed a palladium(0)-catalyzed triple domino process for the regio- and stereoselective synthesis of such olefins.
The team coupled 2-bromoaryl alkynyl biaryls or heteroaryls with norbornene using Pd(PPh3)4 as the catalyst and 1,4-dioxane as the solvent. The reaction involves the formation of three new C–C bonds (pictured red) through double carbopalladation and C–H activation to give a variety of congested tetrasubstituted olefins (example pictured) in good yields. A key step is the formation of a vinylic palladium(II) intermediate via a 5-exo-dig carbopalladation.
The method has a broad substrate scope and tolerates a wide range of substituents. Some of the products have a 9H-pyrrolo[1,2-a]indole core and can act as aggregation-induced emission (AIE) fluorophores.
- Palladium-Catalyzed Synthesis of Tetrasubstituted Olefins by Triple Domino Process,
Kanagaraj Naveen, Savariyappan Albert Nikson, Paramasivan Thirumalai Perumal,
Adv. Synth. Catal. 2017.
DOI: 10.1002/adsc.201700169