The carboxylation of organic halides using CO2 is a basic reaction in organic synthesis. This is usually done using Grignard reagents. Recently, transition-metal-catalyzed carboxylation reactions have attracted considerable attention, however, an excess amount of metallic reductants is needed for this approach.
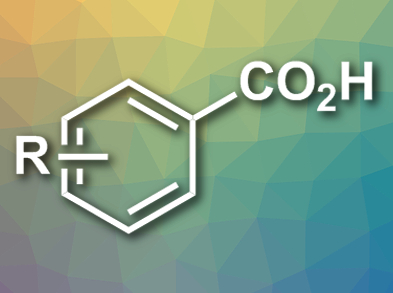
Nobuharu Iwasawa, Tokyo Institute of Technology, Japan, and colleagues have found that aryl bromides and chlorides undergo carboxylation under 1 atm CO2 using Pd(OAc)2 as a carboxylation catalyst and Ir(ppy)2(dtbpy)(PF6) (ppy = 2-phenylpyridine, dtbpy = 4,4’-di-tert-butyl-2,2’-bipyridine) as a photoredox catalyst. N,N-Diisopropylethylamine is used as a non-metallic electron donor and 2-di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl (tBuXPhos) or 2-diphenylphosphino-2′,4′,6′-triisopropylbiphenyl (PhXPhos) are used as ligands. The reactions are carried out in dimethylacetamide (DMA) at room temperature in the presence of Cs2CO3 under visible light irradiation (example product pictured).
The reactions proceed in good yields and tolerate various functional groups; even sterically hindered 2,4,6-triiopropylbromobenzene can be used as a substrate. The reaction is the first example of a Pd-catalyzed carboxylation of aryl chlorides. The proposed mechanism suggests that the photoredox catalysis is involved in the reduction of Pd(II) species to Pd(0) through a successive two-electron transfer.
- Visible Light-Driven Carboxylation of Aryl Halides by the Combined Use of Palladium and Photoredox Catalysts,
Katsuya Shimomaki, Kei Murata, Ruben Martin, Nobuharu Iwasawa,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b04838