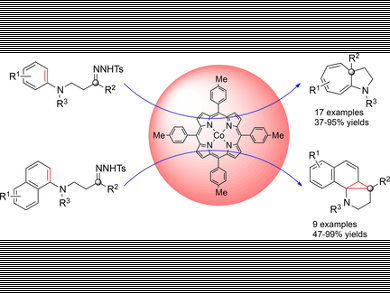
Cycloheptatrienes and norcaradienes are useful building blocks in organic synthesis. They can be efficiently synthesized from transition-metal-catalyzed Buchner reactions and arene cyclopropanations using diazo compounds as carbene sources. Recently, alkyl diazomethanes are found to be effective carbene sources with good diastereo- and chemo-selectivity, but the related studies are still rare.
Chi-Ming Che, The University of Hong Kong, China, and colleagues have developed a method to synthesize cycloheptatriene and norcaradiene derivatives from aniline derived N-tosylhydrozones using cobalt-porphyrin as a catalyst and K2CO3 as a base in dioxane at 105 °C. The reactions gave cycloheptatrienes in good yields. Interestingly, when 2-naphthylamine derivatives were used, the reactions stopped at the cyclopropanation stage to give a tetracyclic product (see pictured).
The reactions are found to be highly regioselective and the cyclopropanation/tautomerization step might not be the rate-determining step. In case of 2-naphthylamine derivatives, the electrocyclic ring opening might be kinetically and thermodynamically disfavored by a concomitant dearomatization. The tetracyclic cyclopropane products can be further converted to other N-heterocycles.
- Cobalt-Porphyrin-Catalyzed Intramolecular Buchner Reaction and Arene Cyclopropanation of In Situ Generated Alkyl Diazomethanes,
Haixu Wang, Cong-Ying Zhou, Chi-Ming Che,
Adv. Synth. Catal. 2017, 359, 2253–2258.
DOI: 10.1002/adsc.201700205