Germanium tetrafluoride (GeF4) is used, e.g., for the preparation of Si-Ge thin films and could have applications in semiconductor technology. Its fluorination product GeF5–, in contrast, had not been isolated so far and only substituted analogues such as [(C2F5)3GeF2]− and [(CF3)3GeF2]− had been characterized. There have been attempts to transform GeF4 to GeF5– using fluorinating agents, e.g., XeF6 or ClO2F, however, the resulting product polymerizes due to insufficient stabilization.
Gašper Tavčar, Jožef Stefan Institute, Ljubljana, Slovenia, and colleagues have synthesized and characterized discrete GeF5– anions. The team used bulky 1,3-bis(2,6-diisopropylphenyl)imidazolium fluoride ([(LDipp)H]F) as a fluorinating agent for GeF4 in acetonitrile. The resulting salt [(LDipp)H][GeF5] was obtained in quantitative yield and characterized using X-ray crystallography as well as Raman and 19F NMR spectroscopy. The researchers successfully performed the same reaction with SiF4 to give [(LDipp)H][SiF5].
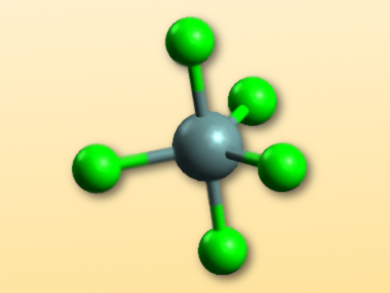
The monomeric MF5– anions (M = Si, Ge) in the products have a trigonal pyramidal structure (pictured). The sterically demanding imidazolium cation stabilizes the discrete anions and protects them from polymerization. In addition, according to quantum chemical calculations, the electrostatic interactions with such large ions favor the trigonal pyramidal structure of GeF5– over the octahedral structure in the polymerized product. According to the researchers, the bulky fluorinating agent used in these syntheses could find widespread use in exploring other areas of fluorine chemistry.
- Discrete GeF5– Anion Structurally Characterized with a Readily Synthesized Imidazolium Based Naked Fluoride Reagent,
Blaž Alič, Melita Tramšek, Anton Kokalj, Gašper Tavčar,
Inorg. Chem. 2017.
DOI: 10.1021/acs.inorgchem.7b01606