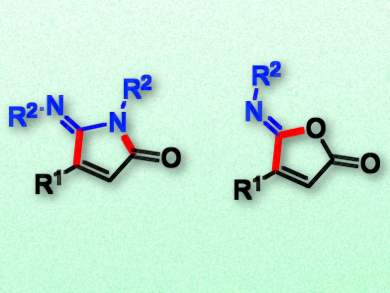
Alkynyl carboxylic acid derivatives can be used as a surrogates for terminal alkynes in organic synthesis, namely in decarboxylative cross-coupling reactions. However, due to the loss of CO2, these reactions are not atom economical.
Wanqing Wu, Huanfeng Jiang, and colleagues, South China University of Technology, Guangzhou, China, have performed cross-coupling reactions of isocyanides and alkynyl carboxylic acids. They combined different isocyanides and alkynyl carboxylic acids with a Pd(OAc)2 catalyst and potassium acetate in either dichloromethane (DCM) or dimethylformamide (DMF) at room temperature.
The team found that, depending on the solvent, the reaction gives either 5-iminopyrrolone derivatives (pictured left, product in DMF) or 5-iminofuranone derivatives (pictured right, product in DCM) in moderate to good yields. According to the researchers, the difference in selectivity is caused by the coordinating nature of DMF. This solvent causes a different preference in an intramolecular acyl transfer step during the catalytic cycle, and thus, a different product.
- Palladium-Catalyzed Cross-Coupling of Alkynyl Carboxylic Acids with Isocyanides: Solvent-Controlled Selective Synthesis of 5-Iminofuranones and 5-Iminopyrrolones,
Weigao Hu, Zun Li, Jiawei Li, Wanqing Wu, Haiyang Liu, Huanfeng Jiang,
Adv. Synth. Catal. 2017.
DOI: 10.1002/adsc.201700533