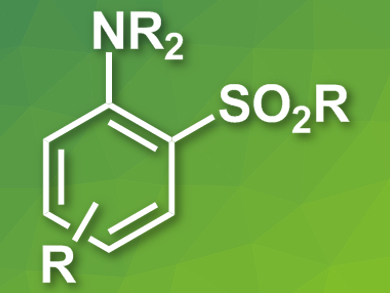
Sulfones are functional groups often found in bioactive compounds. Their synthesis can often involve harsh conditions, which makes the late-stage functionalization of pharmaceutically active molecules difficult.
Darren J. Dixon, Robert S. Paton, Christopher J. Schofield, Martin D. Smith, Michael C. Willis, University of Oxford, UK, and colleagues have developed a mild reaction protocol for the sulfonylation of aniline derivatives with sulfinate salts. The team used [Ir(dF(CF3)ppy)2(dtbpy)]PF6 (dF(CF3)ppy = 3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl, dtbpy = 4,4′-bis(tert-butyl)-2,2′-dipyridyl) as a photoredox catalyst, potassium persulfate as an oxidant, and blue light-emitting diodes (LEDs) to mediate the reaction.
The reaction tolerates a range of substituents on the aromatic ring and proceeds in good yields. The method is scalable and the researchers were able to demonstrate its usefulness for late-stage transformations on drugs like the antibiotic linezolid or the neuroleptic promethazine.
- Direct sulfonylation of anilines mediated by visible light,
Tarn C. Johnson, Bryony L. Elbert, Alistair J. M. Farley, Timothy W. Gorman, Christophe Genicot, Bénédicte Lallemand, Patrick Pasau, Jakub Flasz, José L. Castro, Malcolm MacCoss, Darren J. Dixon, Robert S. Paton, Christopher J. Schofield, Martin D. Smith, Michael C. Willis,
Chem. Sci. 2017.
DOI: 10.1039/c7sc03891g