Functionalized bicyclo frameworks are important building blocks in many natural and bioactive compounds. The palladium-catalyzed intramolecular α-arylation of carbonyl compounds is an attractive strategy to synthesize such skeletons. However, a chiral center at the α-methylene group needs to be preinstalled for the subsequent α-arylation and cyclization steps, which can be very challenging.
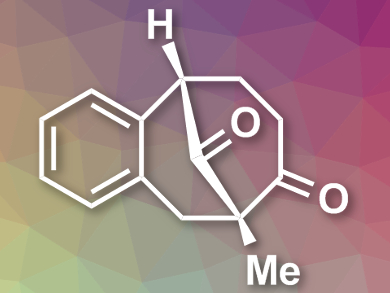
Zhuangzhi Shi, Nanjing University, China, and colleagues have developed an efficient protocol to enantioselectively form two stereogenic centers in one step by using a Pd-catalyzed enantioselective α-arylative desymmetrization of 1,3-diketones. This method can directly construct highly oxygenated and densely substituted chiral bicyclo compounds (example pictured).
The reactions are carried out in toluene at 80 °C in the presence of Cs2CO3 and 4 Å molecular sieves. Pd(OAc)2 was used as the catalyst, together with a ferrocenyl phosphane ligand containing a chiral oxazoline moiety. The protocol can be applied to prepare compounds with different ring sizes as well as products bearing three stereocenters. The researchers demonstrated the utility of the strategy by a short enantioselective total synthesis of (–)-parvifoline.
- Enantioselective Palladium-Catalyzed Intramolecular α-Arylative Desymmetrization of 1,3-Diketones,
Chendan Zhu, Dingyi Wang, Yue Zhao, Wei-Yin Sun, Zhuangzhi Shi,
J. Am. Chem. Soc. 2017, 139, 16486–16489.
DOI: 10.1021/jacs.7b10365