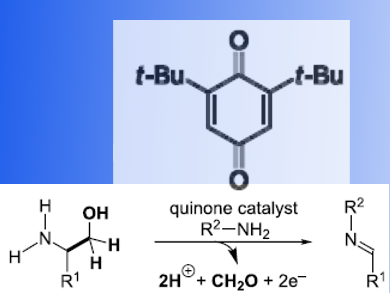
Many synthetic methods exist for the preparation of imines, the very flexible intermediates in organic chemistry. The oldest and most common method for imine synthesis is the condensation of an amine with an aldehyde or ketone. Recently, the catalytic dehydrogenation of amines via cleavage of a C−H bond at the α-position of an amine substrate has gained popularity. C−C bond cleavage at the α-position would allow using renewable resources, such as amino acids and their derivatives, as starting materials. However, this method is not very common.
Michael D. Clift and colleagues, The University of Kansas, Lawrence, KS, USA, have developed an imine synthesis that uses quinone catalysis in an oxidative deformylation of 1,2-amino alcohols to provide N-protected imine products. A wide range of readily accessible amino alcohols and primary amines can be used as starting materials. To demonstrate the synthetic utility of this methodology, the team united in a sequential oxidative deformylation/Mukaiyama−Mannich addition (thio)silyl ketene acetal with 2-phenylglycinol and para-anisidine. In a two-step, one-pot process the β-amino acid derivative was formed in a 60% yield.
According to the researchers, their method demonstrates the synthetic versatility of quinone-catalyzed oxidative C–C bond cleavage. Future work will involve mechanistic studies and the development of catalysts to expand the scope of this chemistry.
- Quinone-catalyzed oxidative deformylation: synthesis of imines from amino alcohols,
Xinyun Liu, Johnny H. Phan, Benjamin J. Haugeberg, Shrikant S. Londhe, Michael D. Clift,
Beilstein J. Org. Chem. 2017, 13, 2895–2901.
https://doi.org/10.3762/bjoc.13.282