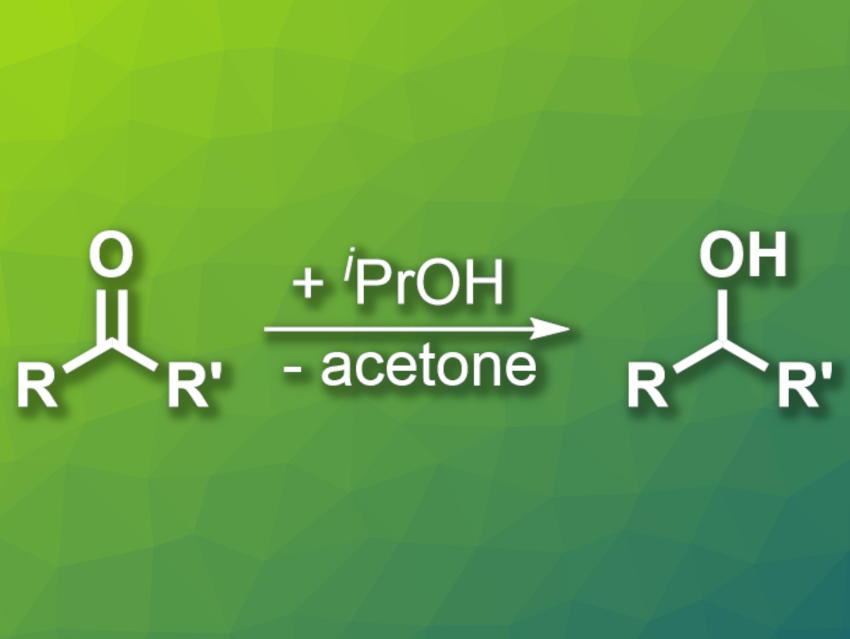
The Meerwein–Ponndorf–Verley (MPV) reduction of ketones and aldehydes uses a sacrificial alcohol to convert carbonyl compounds into the corresponding alcohols (example pictured). There is a variety of catalysts for this reaction, but several of them have drawbacks such as a need for noble metals or a complex preparation. The development of new inexpensive and easy-to-synthesize catalysts can, thus, be useful.
Chao Xie, Hanmin Yang, Zehui Zhang, South-Central Minzu University, Wuhan, China, and colleagues have synthesized a porous zirconium sulfonate via the coordination of Zr(IV) from ZrCl4 onto Amberlyst®-15, a commercially available porous ion-exchange resin with sulfonic acid groups. In the resulting catalyst, the Zr is highly dispersed and coordinated to sulfonate groups.
The team found that the developed catalyst can effectively promote MPV reactions and quantitatively convert carbonyl compounds to the corresponding alcohols, using iPrOH as the sacrificial alcohol. They tested a series of biomass-derived carbonyl compounds as substrates, such as levulinic acid or its esters, with good results. The reaction tolerates reduction-sensitive functional groups such as alkenes or nitro groups. The catalyst could be reused at least five times without an obvious decrease in its catalytic activity.
- Amberlyst-15 supported zirconium sulfonate as an efficient catalyst for Meerwein-Ponndorf-Verley reductions,
Zixin Wang, Chao Xie, Xun Li, Jiabao Nie, Hanmin Yang, Zehui Zhang,
Chem. Commun. 2022.
https://doi.org/10.1039/d2cc00157h