The metal-catalyzed carbofunctionalization of activated alkenes has been studied extensively, but the corresponding transformation of unactivated alkenes is still rare. The Pd-catalyzed carbofunctionalization of unactivated alkenes can be realized by using an amide-linked aminoquinoline (AQ) directing group. However, the strong coordination of the N,N-bidentate AQ group makes the enantioselective reaction challenging. The desired ligand needs to bind the only available site on Pd and accelerate the enantiodetermining step to compete with the background nonasymmetric reaction.
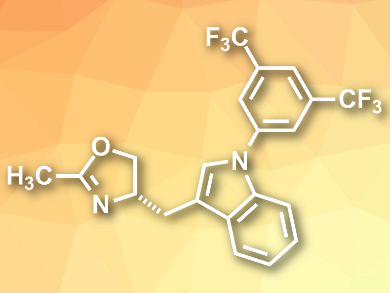
Gong Chen, Nankai University and Collaborative Innovation Center of Chemical Science and Engineering, both Tianjin, China, and Pennsylvania State University, University Park, USA, Gang He, Nankai University and Collaborative Innovation Center of Chemical Science and Engineering, Qian Peng, Nankai University, and colleagues have developed a monodentate oxazoline ligand bearing an N-3,5-di-CF3-phenyl substituted indole (MOXin, pictured). It can be easily prepared in high yield in three steps from low-cost amino acid precursors.
The researchers achieved the first Pd-catalyzed enantioselective hydrocarbofunctionalization of AQ-containing 3-alkenamides with indoles, using Pd(OAc)2 as a catalyst and MOXin as a ligand in the presence of o-phenyl benzoic acid at 60 °C in MeOH. The products were obtained in good to excellent yields and enantioselectivities. The directing group AQ can be removed after the reaction without causing a racemization of the products.
- Palladium-Catalyzed Amide-Directed Enantioselective Hydrocarbofunctionalization of Unactivated Alkenes Using a Chiral Monodentate Oxazoline Ligand,
Hao Wang, Zibo Bai, Tangqian Jiao, Zhiqiang Deng, Huarong Tong, Gang He, Qian Peng, Gong Chen,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b00641