The use of a second cooperative catalyst together with N-heterocyclic carbene (NHC) organocatalysts can broaden the substrate scope and enable fundamentally new reactions. Lewis or Brønsted acids have been often used as the cooperative catalyst in this way.
Transition metals (TM), in contrast, have been rarely used with an NHC in a cooperative fashion. The reason is that the carbenes tend to bind with the TM and, therefore, lose their individual reactivity. If the ligation between NHC and TM can be prevented, each catalyst can be separately fine-tuned. This could significantly help the development of new cooperative catalytic systems.
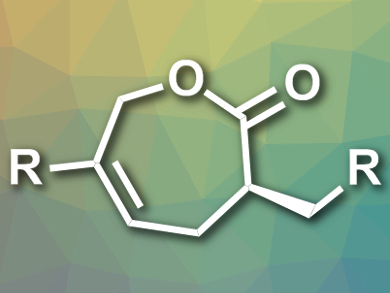
Frank Glorius, University of Münster, Germany, and colleagues have developed an NHC/Pd cooperative catalytic system by adding the strong chelating bidentate ligand (R)-Tol-BINAP, (R)- 2,2′-bis(di-p-tolylphosphino)-1,1′-binaphthyl, to prevent the binding of NHC to the TM. This catalytic system was successfully applied in an enantioselective [5+2] annulation of enals and vinylethylenes. The reactions are carried out in toluene at room temperature in the presence of N-methylpiperidine and the products, ε-caprolactones (pictured), are obtained in good yields and excellent enantioselectivity.
- Highly Enantioselective [5 + 2] Annulations through Cooperative N-Heterocyclic Carbene (NHC) Organocatalysis and Palladium Catalysis,
Santanu Singha, Tuhin Patra, Constantin G. Daniliuc, Frank Glorius,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b00868