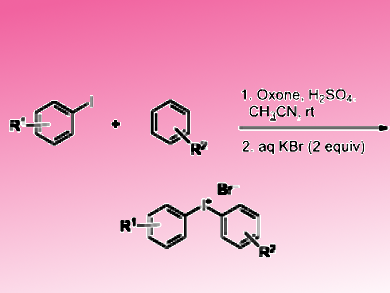
Diaryliodonium salts, also known as diaryl-λ3-iodanes, have broad synthetic application as electrophilic arylating reagents in reactions with various nucleophiles including electron-rich carbon-centered species. The development of novel synthetic approaches to diaryliodonium salts based on the use of inexpensive, commercially available and easy-to-use oxidants is important and challenging.
Thomas Wirth, Cardiff University, Cardiff, UK, Mekhman S. Yusubov, The Tomsk Polytechnic University, Tomsk, Russia, and colleagues have developed a facile synthesis of diaryliodonium salts from various aryl iodides and arenes using the readily available and effective oxidant Oxone® (2KHSO5·KHSO4·K2SO4) and sulfuric acid (pictured). The addition of aqueous KBr to the reaction mixture is necessary as the high solubility of the diaryliodonium sulfonates does not allow their isolation from the reaction mixture. Adding acetonitrile resulted in an increased yield.
The procedure allows the synthesis of a wide range of iodonium salts in good yields containing electron-donating and electron-withdrawing substituents. Particularly attractive is the possibility of the one-pot synthesis of symmetric bis-aryl iodonium salts directly from arenes via an iodination–oxidation sequence.
- One-pot synthesis of diaryliodonium salts from arenes and aryl iodides with Oxone–sulfuric acid,
Natalia Soldatova, Pavel Postnikov, Olga Kukurina, Viktor V. Zhdankin, Akira Yoshimura, Thomas Wirth, Mekhman S. Yusubov,
Beilstein J. Org. Chem. 2018, 14, 849–855.
https://do.org/10.3762/bjoc.14.70