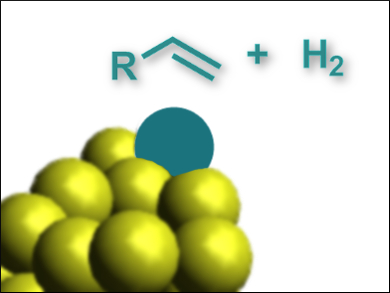
Gold clusters which are stabilized by poly(N-vinyl-2-pyrrolidone) (Au:PVP) are promising for applications in catalysis. Small structural and electronic changes can improve their catalytic activity significantly. Doping the clusters with other metals, such as Ag or Pd, can induce such improvements.
Tatsuya Tsukuda, University of Tokyo and Kyoto University, both Japan, and colleagues have found that a single rhodium atom can turn a catalytically inactive Au34 cluster into a useful catalyst for the hydrogenation of olefins. The team prepared doped clusters by mixing solutions of HAuCl4 and RhCl3 in a molar ratio of 97:3 and combining them with NaBH4 in the presence of PVP at 0 °C. Mass spectrometry showed that the dominant product is a cluster with a single Rh atom, Au34Rh1. For comparison, the researchers also synthesized an Au34 cluster and a variant with a single Pd atom, Au33Pd1.
The team found that the undoped Au34 cluster is inactive for the hydrogenation of three different olefins, with conversion rates below 1 %. The Pd-variant has a slightly improved catalytic activity with conversion rates of 2–33 %, and the Rh-doped cluster has a high activity with conversion rates of 83–95 %. According to the researchers, this is caused by the presence of an exposed Rh atom with a low coordination state on the cluster surface, which acts as the active site for catalysis.
- Prominent hydrogenation catalysis of a PVP-stabilized Au34 superatom provided by doping a single Rh atom,
Shingo Hasegawa, Shinjiro Takano, Seiji Yamazoe, Tatsuya Tsukuda,
Chem. Commun. 2018, 54, 5915–5918.
https://doi.org/10.1039/c8cc03123a