CO2 is a greenhouse gas and a major contributor to climate change. Capturing CO2 and converting it to useful chemicals is one possible approach to control its atmospheric levels. Usually, the capture and the chemical conversion require two separate process steps. In addition, the capture step needs to work at low concentrations of CO2 from the atmosphere or exhaust gas.
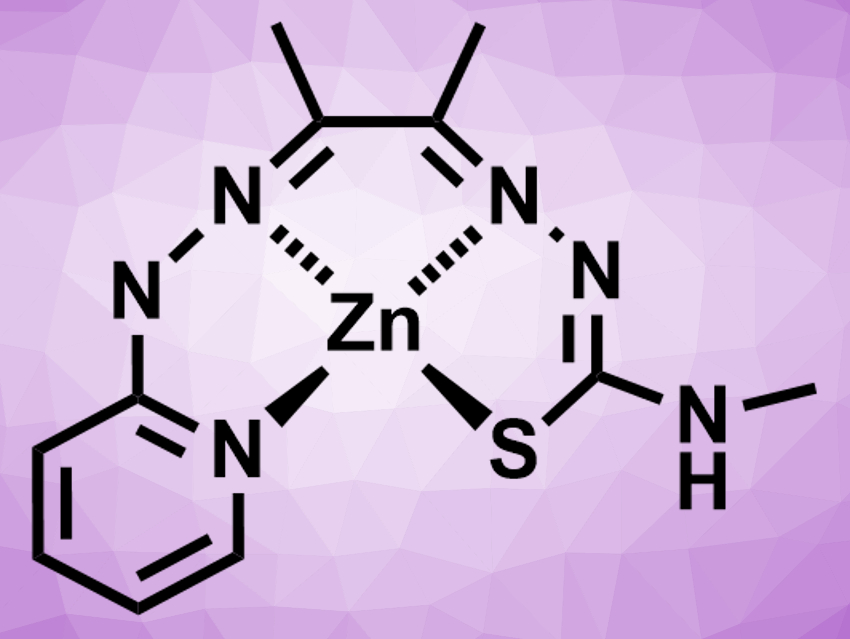
Craig A. Grapperhaus, University of Louisville, KY, USA, and colleagues have developed a method that combines these two steps, based on a zinc(II) complex with a redox-active ligand. The catalyst is Zn(DMTH) (pictured, DMTH = diacetyl-2-(4-methyl-3-thiosemicarbazonate)-3-(2-pyridinehydrazonato)). It promotes the reduction of CO2 from air to give formate (HCO2–). In methanol, the complex is converted to the active catalyst Zn(HDMTH)(OCH3). This species can fix CO2 from air and form the methylcarbonate intermediate Zn(HDMTH)(CO3CH3). In this compound, CO2 is activated and can react with hydride to give the desired formate and restore methanol and Zn(DMTH).
The ligand–metal interaction helps to activate the CO2 by acting like a frustrated Lewis pair (FLP). FLPs contain a Lewis acid and a Lewis base that cannot combine to form an adduct due to steric hindrance. Many of them can activate small molecules. In the Zn-based catalyst, there is an FLP-like interaction between the Zn(II) ion and the non-coordinating nitrogen of the 2-pyridinehydrazonato group. According to the researchers, the work provides a new approach to CO2 reduction catalysis.
- Exploiting Metal–Ligand Cooperativity to Sequester, Activate, and Reduce Atmospheric Carbon Dioxide with a Neutral Zinc Complex,
Steve P. Cronin, Jacob M. Strain, Mark S. Mashuta, Joshua M. Spurgeon, Robert M. Buchanan, Craig A. Grapperhaus,
Inorg. Chem. 2020.
https://doi.org/10.1021/acs.inorgchem.0c00121