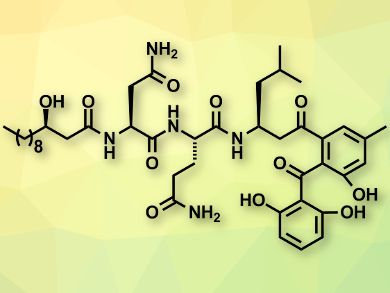
The natural products asperphenins A and B (latter pictured above) were isolated from a marine-derived fungus collected from the shore of a Korean Island. The two compounds differ only in the configuration of one stereocenter. Both have shown significant cytotoxicity against human cancer cell lines and could serve as lead molecules for the development of new chemotherapy drugs.
Yian Guo, Tao Ye, Peking University, Shenzhen, China, and colleagues have achieved the first total synthesis of asperphenins A and B, using a convergent approach. The team started from commercially available compounds and synthesized the first fragment of the target molecule using an enantioselective aldol reaction with caprinaldehyde and two consecutive peptide coupling reactions.
The second fragment was prepared using a Grignard reaction and a subsequent oxidation to give an aldehyde. This aldehyde was coupled with a phosphorane derived from a trifluoroacetamide derivative in a Wittig reaction, giving an internal alkene. A modified, mild Wacker oxidation allowed the researchers to convert this alkene to the ketone found in the final products.
The two fragments of the target molecules were finally combined using an amide formation. The complete synthesis involved 15 steps for both asperphenin A and B, with overall yields of 9.7 % and 14.2 %, respectively.
- Total Synthesis of Asperphenins A and B,
Jia-Lei Yan, Yingying Cheng, Jing Chen, Ranjala Ratnayake, Long H. Dang, Hendrik Luesch, Yian Guo, Tao Ye,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b02652