Nanoparticles can be used to build larger analogues of molecules, so-called colloidal molecules (CMs). Nanoparticles with four tetrahedrally arranged “dimples” or patches, for example, can bind four other particles to give methane analogues.
Serge Ravaine and colleagues, University of Bordeaux, France, have prepared patchy silica nanoparticles with either two or four dimples. These were used as colloidal atoms (CAs) that simulate sp- or sp3-hybridization, respectively. The team first used silica seeds to grow silica nanospheres. These were then used for a seeded-growth emulsion polymerization of styrene, which gave silica particles surrounded by either two or four polystyrene (PS) satellite nodules, depending on the size of the seed. The PS was then mostly dissolved to create dimples in the particle surface, and the residual PS was functionalized with amine groups.
To create the satellite particles that act as, e.g., hydrogen or halogen atom equivalents and bond to these silica cores, the team first prepared gold nanoparticles. These were surface-functionalized with PEG-SH (O-[2-(3-mercaptopropionylamino)ethyl]-O‘-methylpoly(ethylene glycol)) and then coated with silica. Finally, the satellites were functionalized with carboxylic acid groups. Satellites without a gold core were also prepared to simulate a second type of CA and allow the synthesis of asymmetric CMs.
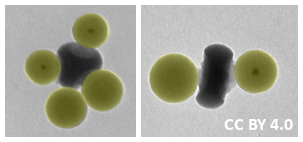
The researchers prepared CM analogues of, for example, CF4, CBrF3, or CBr2F2 (pictured left below), by combining sp3-type cores with the corresponding ratio of satellite particles. The satellites each attach to one “dimple” by an amide bond formed between the amine on the core and the carboxylic acid on the satellite. Similarly, linear analogues of, e.g., BeF2 or BeBrF (pictured right below) were synthesized using an sp-type core. According to the team, the developed approach could be useful to create nanostructured building blocks for functional materials.
 |
|
Source: Beilstein J. Nanotechnol., © 2018 Rouet et al., CC BY 4.0. |
- Colloidal chemistry with patchy silica nanoparticles,
Pierre-Etienne Rouet, Cyril Chomette, Laurent Adumeau, Etienne Duguet, Serge Ravaine,
Beilstein J. Nanotechnol. 2018, 9, 2989–2998.
https://doi.org/10.3762/bjnano.9.278



