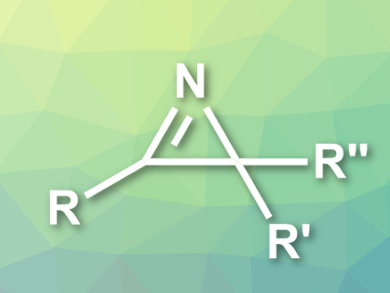
2H-Azirines (pictured) are heterocycles with useful reactivities for a range of organic syntheses. Their ring structure also occurs in biologically active compounds. They can be prepared in an atom-economical manner by the oxidative cyclization of enamines.
Wenquan Yu, Junbiao Chang, and colleagues, Zhengzhou University, China, have developed a metal-free oxidative cyclization of enamines under mild conditions, mediated by molecular iodine. The team used various enamines as substrates, which gave the desired substituted 2H-azirines in the presence of I2 as an oxidant, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as a base, and dichloromethane as the solvent. The reaction proceeds at room temperature.
The desired 2H-azirines were obtained in moderate to excellent yields and the reaction can be performed at the gram scale. The team proposes a reaction mechanism involving a iodination of the enamine, followed by a proton abstraction. An attack of the imine nitrogen atom on the iodo-substituted carbon then forms the ring, and another proton abstraction gives the desires 2H-azirine. According to the researchers, the developed method is an attractive approach for 2H-azirine synthesis under mild reaction conditions.
- Synthesis of 2H-Azirines via Iodine-Mediated Oxidative Cyclization of Enamines,
Manman Wang, Jiao Hou, Wenquan Yu, Junbiao Chang,
J. Org. Chem. 2018.
https://doi.org/10.1021/acs.joc.8b02022


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
