Borylenes are the boron analogue of carbenes. They are even more reactive and difficult to stabilize than the already generally short-lived carbenes. Diatomic borylenes of the type XB: (X = H, F, Cl, Br, I) are particularly unstable. Fluoroborylenes (FB:), for example, are very rare and had only been synthesized with metal-based complex fragments in their coordination sphere so far.
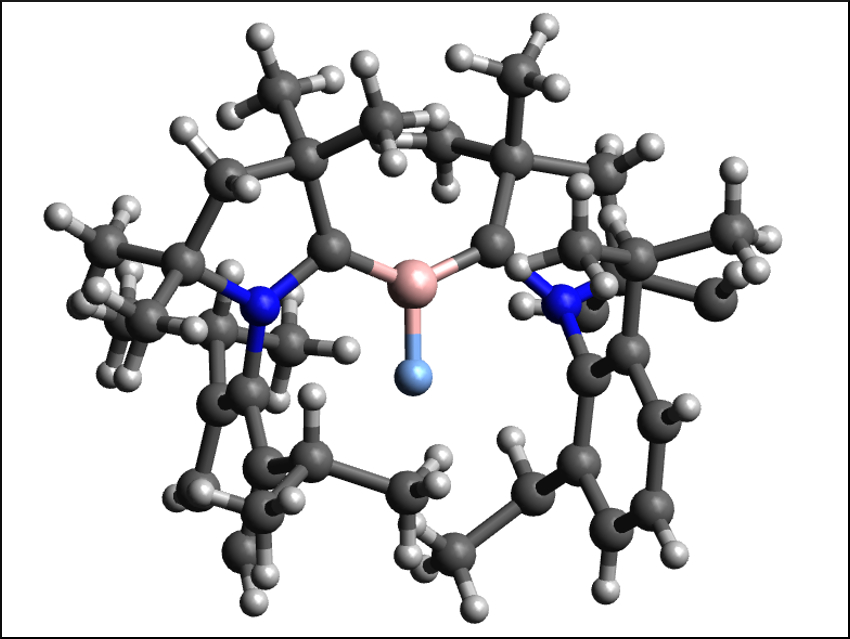
Dietmar Stalke, University of Göttingen, Germany, and Indian Institute of Science, Bangalore, Wolfgang Kaim, University of Stuttgart, Germany, Eluvathingal D. Jemmis, Indian Institute of Science, Herbert W. Roesky, University of Göttingen, and colleagues have synthesized and characterized a metal-free fluoroborylene, (Me-cAAC)2BF (pictured), stabilized by cyclic (alkyl)(amino) carbenes (cAACs). The team prepared the desired compound from (Me-cAAC)BF3 and Me-cAAC using potassium graphite (KC8) in toluene at −78 °C.
The carbene-stabilized fluoroborylene was isolated in 78 % yield as red-violet crystals. The compound was fully characterized and its composition confirmed using 1H, 13C, 11B, and 19F NMR spectroscopy, mass spectrometry, single crystal X-ray diffraction, and elemental analysis. The researchers also obtained the corresponding radical cation, [(Me-cAAC)2BF]˙+, via a one-electron oxidation with LiB(C6F5)4. According to the team’s density functional theory (DFT) calculations, the fluoroborylene is stabilized by the delocalization of the electrons from boron over the cAAC ligands.
- Isolation of base stabilized fluoroborylene and its radical cation,
Samir Kumar Sarkar, Mujahuddin M. Siddiqui, Subrata Kundu, Munmun Ghosh, Johannes Kretsch, Peter Stollberg, Regine Herbst-Irmer, Dietmar Stalke, A. Claudia Stückl, Brigitte Schwederski, Wolfgang Kaim, Sagar Ghorai, Eluvathingal D. Jemmis, Herbert W. Roesky,
Dalton Trans. 2019.
https://doi.org/10.1039/c9dt01899a