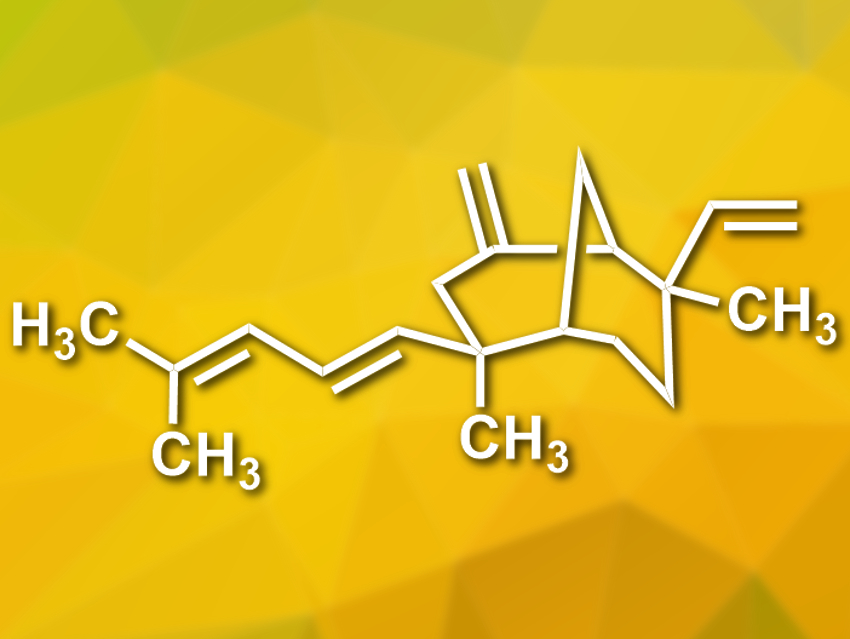
Xishacorenes A, B, and C are natural products with a diterpenoid structure. They were isolated from a coral found off the coast of the Xisha Islands in the South China Sea. The compounds were obtained together with the structurally related fuscol. It has been proposed that fuscol is a precursor in the biosynthesis of the xishacorenes.
Richmond Sarpong, University of California, Berkeley, USA, and colleagues have performed a bioinspired synthesis of the xishacorenes starting from fuscol. In addition to the known xishacorenes A, B, and C, they found a fourth, previously unknown member of this family of compounds, which they named xishacorene D (pictured). They reacted fuscol with BF3·OEt2 at –78 °C and obtained a mixture of xishacorenes with a combined yield of 49 %. The products were separated using chromatography.
According to the researchers, the desired natural products are formed via an acid-catalyzed cyclization of fuscol, which gives the bicyclic system. They propose that the newly discovered xishacorene D might also occur in nature alongside the other variants.
- Bio-inspired synthesis of xishacorenes A, B, C, and a new congener from fuscol,
Alexander R. Rovira, Nicolas Müller, Weiwen Deng, Chudi Ndubaku, Richmond Sarpong,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc02572c