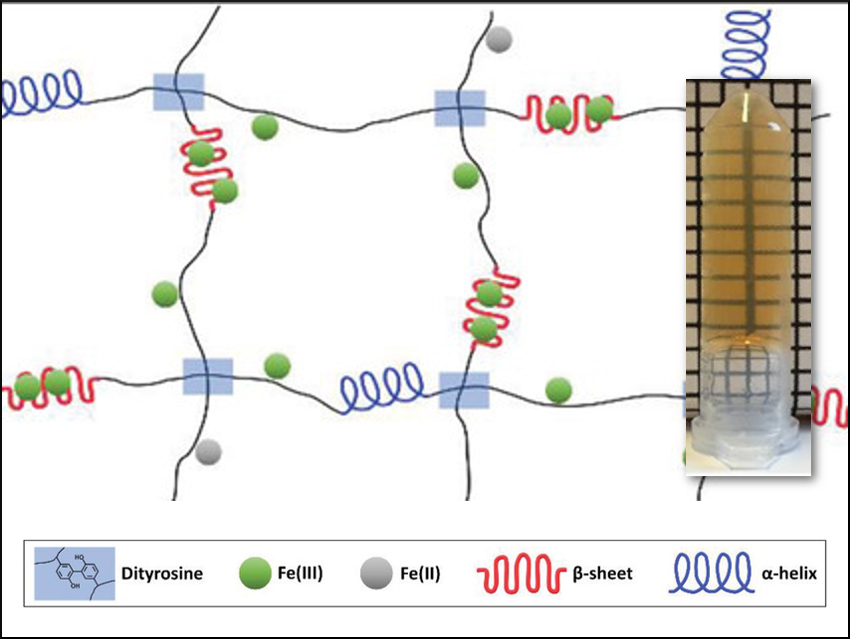
Many proteins or polymers can be crosslinked to form hydrogels, which can be used, e.g., in medicine. Often, enzymes are used for the preparation of such hydrogels, for example, from hyaluronic acid, gelatin, alginate, chitosan, or silk fibroin. However, some of the enzyme remains in the hydrogel products. This can cause an immunological response in the body.
David L. Kaplan, Tufts University, Medford, MA, USA, and colleagues have developed a method for the enzyme-free synthesis of chemically crosslinked silk fibroin hydrogels for biomedical applications. Silk fibroin protein is promising for medicine due to its biocompatibility, controllable biodegradability, mechanical strength, and mild immunological response. The team used a Fenton reaction to chemically crosslink the fibroin. This reaction is based on a redox reaction between Fe2+ and H2O2 that generates a reactive hydroxyl radical (•OH). These radicals initiate crosslinking reactions, in this case, the formation of dityrosine bonds in the protein through the oxidation of tyrosine residues.
The Fe3+ ions formed during the reaction can interact with silk fibroin and trigger the formation of β‐sheet structures (pictured in red). These β-sheet structures scatter light and make the hydrogel opaque. If the reaction is performed in the presence of the iron-chelating agent ethylenediaminetetraacetic acid (EDTA), fewer Fe3+ ions can interact with the protein and the hydrogel remains transparent.
This can be important for applications in ophthalmology, optical sensing, and diagnostics. According to tests on mouse cells, the hydrogels prepared by the Fenton reaction are biocompatible.
- Silk Hydrogels Crosslinked by the Fenton Reaction,
Jaewon Choi, Meghan McGill, Nicole R. Raia, Onur Hasturk, David L. Kaplan,
Adv. Healthcare Mater. 2019.
https://doi.org/10.1002/adhm.201900644