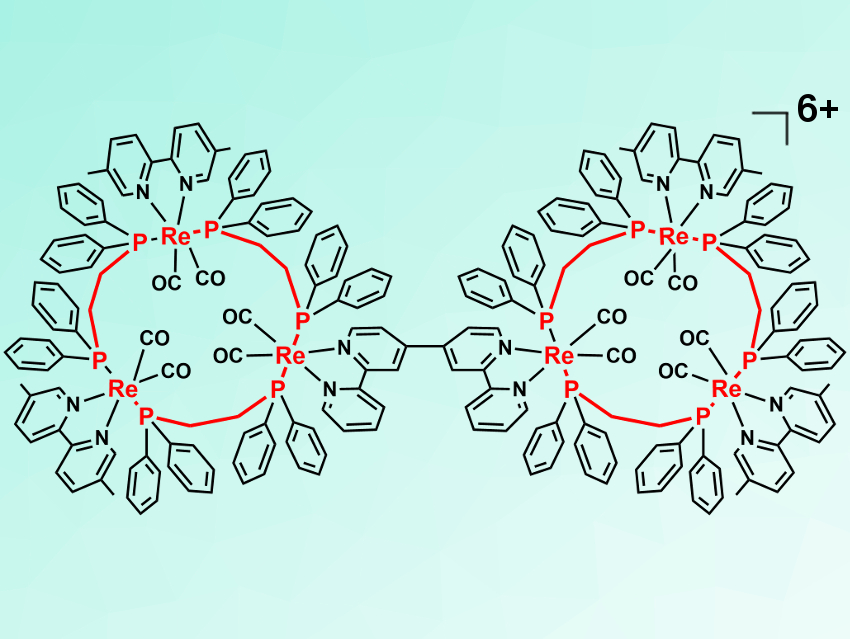
Ring-shaped molecules are found in important photoactive molecules such as chlorophyll. Ring-shaped multinuclear Re(I) complexes can also be photoactive. Some of these compounds provide long emission lifetimes and tunable properties. They can, for example, be useful as a photosensitizer in catalytic reactions.
Yasuomi Yamazaki and Osamu Ishitani, Tokyo Institute of Technology, Japan, have coupled two ring-shaped trinuclear Re(I) complexes using a homocoupling reaction between two rings, each functionalized with a bromo group. The team first synthesized a bromo-functionalized linear trinuclear complex, which was cyclized with a bidentate phosphine ligand to give the desired trinuclear rings. These rings were then coupled using a palladium catalyst to give the desired hexanuclear complex (pictured)—shaped like a pair of glasses—in a yield of 31 %.
The hexanuclear complex provides an efficient intramolecular energy transfer and accumulates the excitation energy from light in the central Re(I) units near the bridging ligand. Thus, it has suitable properties to be used as a light-harvesting system.
- Synthesis of an Emissive Spectacle-Shaped Hexanuclear Rhenium(I) Complex,
Yasuomi Yamazaki, Osamu Ishitani,
Inorg. Chem. 2019.
https://doi.org/10.1021/acs.inorgchem.9b01856