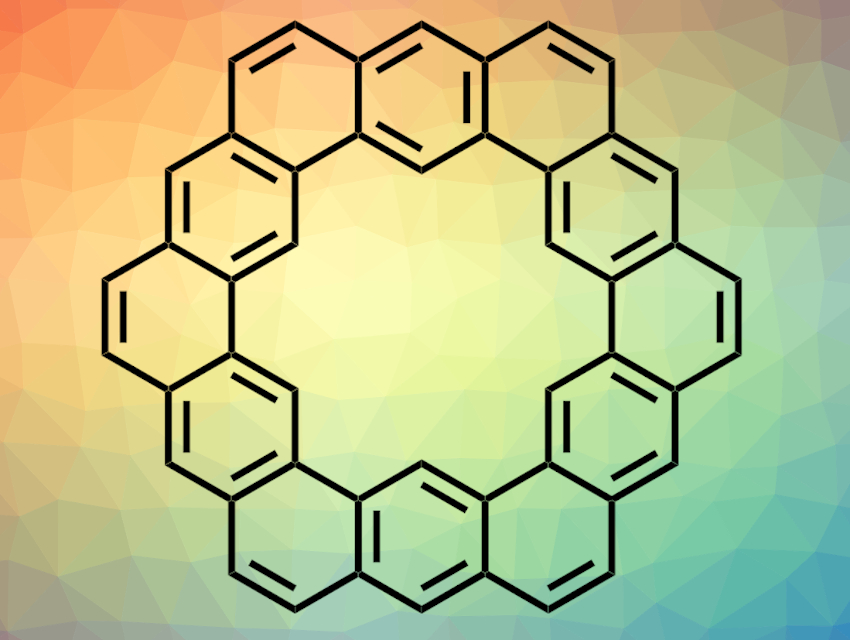
The cycloarene kekulene was synthesized for the first time in 1978 [1] and has been the subject of many theoretical studies. Two different models for the electronic structure of kekulene have been proposed. One model describes a completely delocalized π-electron distribution in kekulene, the other (the so-called Clar model, pictured) assumes an alternation between 2- and 6-π-electron systems.
Diego Peña, Dolores Pérez, Universidade de Santiago de Compostela, Spain, and colleagues have synthesized kekulene using a new approach for generating the pentacyclic building block 5,6,8,9-tetrahydrobenzo[m]tetraphene. They used a double Diels–Alder reaction between two molecules of styrene and an aryne. The aryne was generated by a fluoride-induced elimination from an aryl bistriflate. With this method, the team obtained the desired pentacyclic building block in one step from commercially available precursors. Two of these building blocks were then connected to form kekulene.
Kekulene was analyzed by atomic force microscopy (AFM) and theoretical calculations were carried out. The brightness and length of the bonds in the AFM images indicate that the bond orders in the molecule are in accordance with the Clar model. The results of the calculations also favor this structure. According to these results, the Clar model is the more accurate description of kekulene’s structure.
- Revisiting Kekulene: Synthesis and Single-Molecule Imaging,
Iago Pozo, Zsolt Majzik, Niko Pavliček, Manuel Melle-Franco, Enrique Guitián, Diego Peña, Leo Gross, Dolores Pérez,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b07926
Reference
- [1] Benzenoid versus Annulenoid Aromaticity: Synthesis and Properties of Kekulene,
François Diederich, Heinz A. Staab,
Angew. Chem. Int. Ed. Engl. 1978, 17, 372–374.
https://doi.org/10.1002/anie.197803721