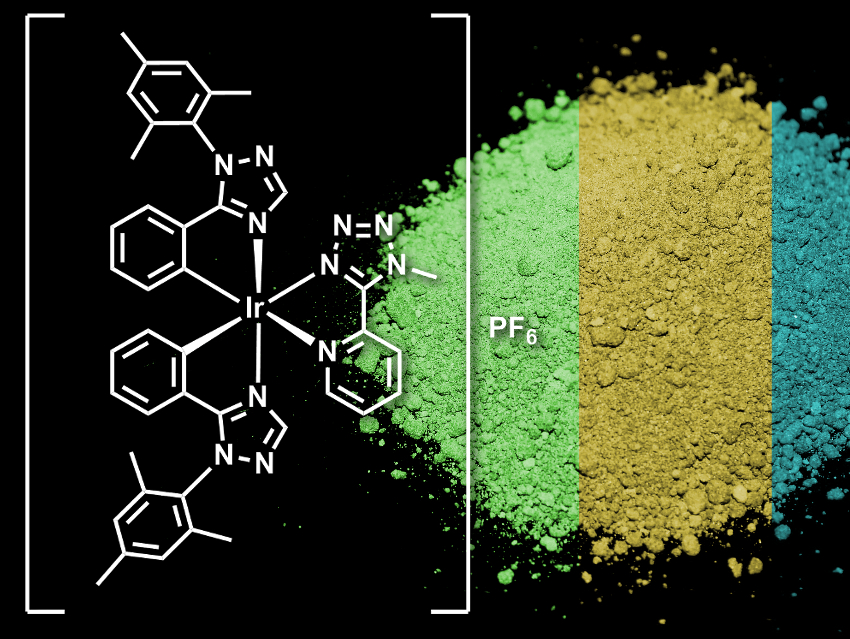
Piezochromic luminescent (PCL) materials can change their light-emission properties upon mechanical stimuli. Usually, they can be switched between two polymorphs and, thus, colors. Materials with three or more luminescent colors are rare.
Dongxia Zhu, Zhongmin Su, Northeast Normal University, Changchun, China, Martin R. Bryce, Durham University, UK, and colleagues have synthesized an iridium complex that can switch between blue, green and yellow luminescent states. The complex, [Ir(Mesptz)2Mtzpy]+ (pictured, Mesptz = 1-mesityl-5-phenyl-1H-1,2,4-triazole, Mtzpy = 2-(1-methyl-1H-tetrazol-5-yl)pyridine), was synthesized from IrCl3·H2O, Mesptz, and Mtzpy. It was used in the form of its PF6− salt.
The complex was specifically designed to be a multicolor PCL material. The team used mesityl substituents to cause a crystal with large intermolecular distances that can “collapse” into an amorphous form under mechanical pressure. They also included heterocyclic groups, which can favor polymorphism.
In the solid state, the compound exists in two crystalline polymorphs and an amorphous form. These three different forms luminesce in three different colors: one crystalline form in blue, the amorphous form in yellow, and the second crystalline form in green. The forms can be reversibly converted into each other by mechanical grinding or by adding small amounts of either toluene or ethanol. According to the researchers, this is the first time three-color luminescence switching has been observed in an Ir(III) complex.
- Reversible tricolour luminescence switching based on a piezochromic iridium(iii) complex,
Tianzhi Yang, Yue Wang, Xingman Liu, Guangfu Li, Weilong Che, Dongxia Zhu, Zhongmin Su, Martin R. Bryce,
Chem. Commun. 2019.
https://doi.org/10.1039/c9cc08545a