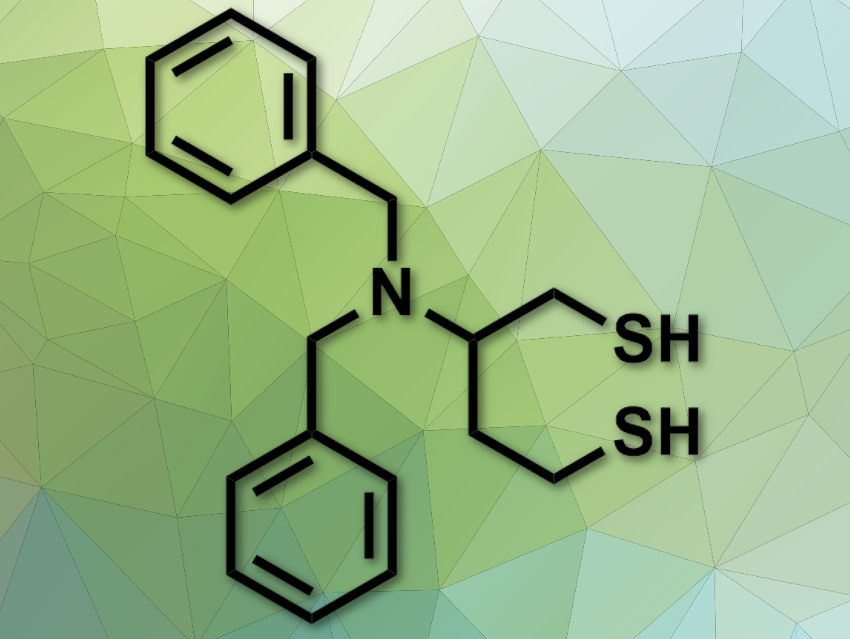
The conversion between dithiols and disulfides is important in biochemistry. Disulfide-reducing agents are widely used in this field, either to protect free thiols or reduce disulfide bridges in peptides. However, commonly used reducing agents, such as β-mercaptoethanol (β-ME), dithiothreitol (DTT), or tris(2-carboxyethyl)phosphine (TCEP) have issues: some smell very bad, some do not work well at physiological pH levels, and some are only usable in aqueous solvents.
Fernando Albericio, University of KwaZulu-Natal, Durban, South Africa, University of Barcelona and Networking Centre on Bioengineering, Biomaterials, and Nanomedicine, Barcelona, Spain, Beatriz G. de la Torre, University of KwaZulu-Natal, and colleagues have developed a new disulfide-reducing agent, 2-(dibenzylamino)butane-1,4-dithiol (DABDT, pictured). This reagent was synthesized from low-cost aspartic acid in five steps.
DABDT is soluble in several organic solvents, e.g., in N,N-dimethylformamide (DMF), dichloromethane, tetrahydrofuran (THF), ethyl acetate, acetonitrile, and methanol. It has a good reducing capacity, is stable in solid form, and has no unpleasant smell.
- 2-(Dibenzylamino)butane-1,4-dithiol (DABDT), a Friendly Disulfide-Reducing Reagent Compatible with a Broad Range of Solvents,
Sinenhlanhla N. Mthembu, Anamika Sharma, Fernando Albericio, Beatriz G. de la Torre,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b04106