Intermetallic compounds and chalcogenides are two very different types of compounds: the former feature metallic bonding and electric conductivity, while the latter are semiconductors with covalent and/or ionic bonds. Metal-rich chalcogenides, or subchalcogenides, fall between these two extremes. Depending on the amount and type of metals, these compounds can be insulators, semiconductors, or semimetals.
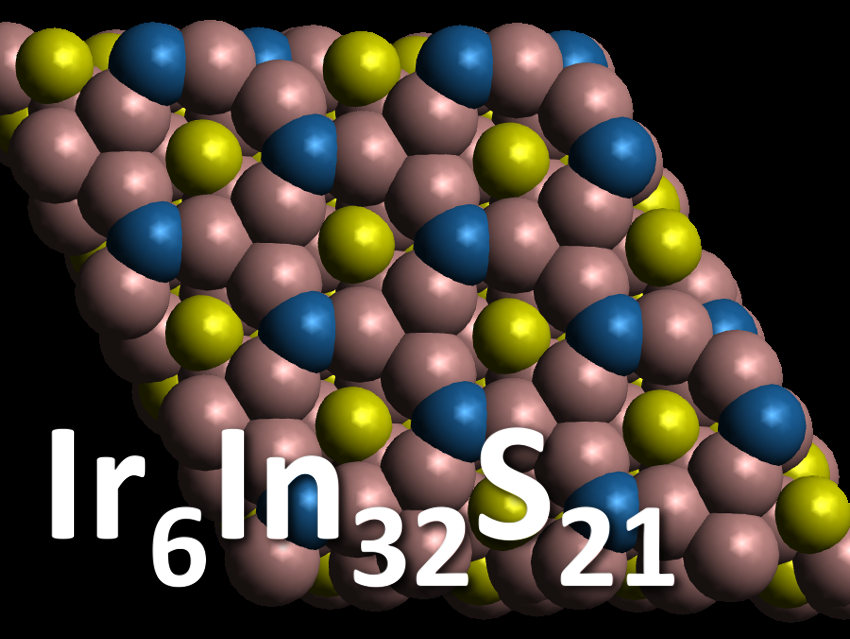
Mercouri G. Kanatzidis, Northwestern University, Evanston, IL, USA, and colleagues have synthesized a new semiconducting subchalcogenide compound, Ir6In32S21. The compound was synthesized from solid sulfur and iridium powder in liquid indium at 1000 °C. It was characterized using single-crystal X-ray diffraction. The team found that the compound crystallizes in a new structure type (pictured) in the polar P31m space group. The crystals contain a honeycomb-like structure made up of distorted, singly capped IrIn7 trigonal prisms and distorted IrIn6 octahedra. The sulfur is bonded exclusively to indium in the form of In/S tetrahedra. Indium thus acts as a bridge between its more electronegative counterparts.
Ir6In32S21 is semiconducting with a band gap of 1.48 eV. According to the researchers, this shows that extended metal–metal bonded networks can still lead to semiconducting behavior. The work could provide a basis for a better understanding of semiconducting behavior in subvalent materials.
- Ir6In32S21, a polar, metal-rich semiconducting subchalcogenide,
Jason F. Khoury, Jiangang He, Jonathan E. Pfluger, Ido Hadar, Mahalingam Balasubramanian, Constantinos C. Stoumpos, Rui Zu, Venkatraman Gopalan, Chris Wolverton, Mercouri G. Kanatzidis,
Chem. Sci. 2020.
https://doi.org/10.1039/c9sc05609b