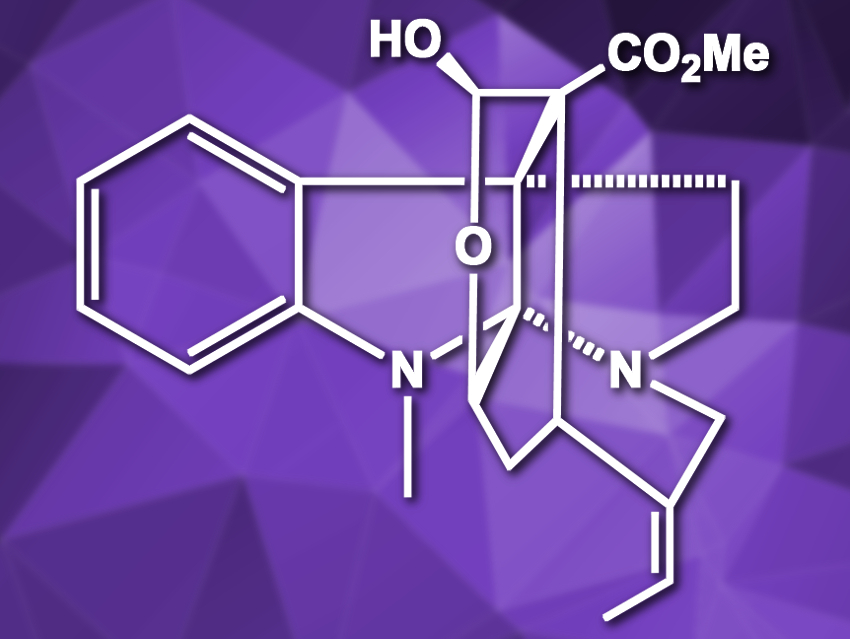
Akuammiline alkaloids are a family of natural products with a pentacyclic framework. They show interesting pharmaceutical activities, but are difficult to synthesize due to their complexity. Corymine (pictured) and deformylcorymine are two members of this family which differ in the functionalization of the cyclohexane ring.
Chaozhong Li, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and Ningbo University of Technology, China, and colleagues have performed the first enantioselective total synthesis of (+)-corymine and (−)-deformylcorymine. The team started from commercially available N-nosyltryptamine and obtained (+)-corymine in 11 steps and 3.6 % overall yield. (−)-Deformylcorymine was also synthesized in 11 steps, with 3.1 % overall yield.
The team used a copper-catalyzed enantioselective addition of dimethyl malonate to a 3-bromooxindole to create a key stereocenter with four carbon substituents. Another key transformation was an intramolecular nucleophilic C- and N-addition that was used to create the cyclohexane and the pyrrolidinyl rings. The seven-membered azepanyl ring was prepared via a nickel-catalyzed 7-endo cyclization. The synthetic strategy could also be used to prepare other akuammiline alkaloids.
- Enantioselective Total Synthesis of (+)-Corymine and (−)-Deformylcorymine,
Benxiang Zhang, Xiaoqing Wang, Chaozhong Li,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c00302