Antiviral drugs that target a variety of viruses, i.e., broad-spectrum antivirals, could be particularly useful to treat rare or new virus strains, such as the one currently causing the outbreak of COVID-19. This approach could be more commercially viable than developing a drug for every single disease caused by viruses and, thus, improve treatment options. To achieve broad activity, the drugs need a target that several viruses share. The very common enteroviruses, for example, depend on viral proteases to multiply. These proteases have similar active sites to the equivalent proteases in rarer coronaviruses, such as the SARS and MERS viruses, even though the viruses are very different overall.
Hong Liu, Shanghai Institute of Materia Medica, China, Rolf Hilgenfeld, Shanghai Institute of Materia Medica and University of Lübeck, Germany, and colleagues have developed α-ketoamides that could inhibit these proteases in coronaviruses and enteroviruses and, thus, prevent the replication of the viruses. The team used structure-based design, i.e., they analyzed crystal structures of the target proteases and of protease–inhibitor complexes. These structures were used to guide the optimization of the substituents at the α-ketoamides.
The researchers found that the best inhibitors have a γ-lactam derivative of glutamine and a cyclopentylmethyl or cyclohexylmethyl group next to the α-ketoamide unit. These inhibitors showed good activity against both enteroviruses and coronaviruses in cell cultures. The compounds showed only weak cytotoxicity. According to the team, due to the high similarity between the main proteases of the SARS virus and the new coronavirus that causes COVID-19, the developed inhibitors could also show antiviral activity against the new coronavirus.
- α-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment,
Linlin Zhang, Daizong Lin, Yuri Kusov, Yong Nian, Qingjun Ma, Jiang Wang, Albrecht von Brunn, Pieter Leyssen, Kristina Lanko, Johan Neyts, Adriaan de Wilde, Eric J. Snijder, Hong Liu, Rolf Hilgenfeld,
J. Med. Chem. 2020.
https://doi.org/10.1021/acs.jmedchem.9b01828
Also of Interest
- Clever Picture: Coronavirus Entering and Replicating in a Host Cell,
Vera Koester,
ChemViews Mag. 2020.
https://doi.org/10.1002/chemv.202000018
Where the coronavirus comes from and how it infects the human body - Structure of “Spike” Protein in New Coronavirus Determined,
ChemistryViews.org 2020.
Results could help to develop a vaccine and antibody treatments - Antiviral Drug Shows Promise Against MERS Coronavirus,
ChemistryViews.org 2020.
The broad-acting drug remdesivir could also be useful against the current coronavirus disease (COVID-19) - How Long Can Coronaviruses Live on Surfaces?,
ChemistryViews.org 2020.
Literature review finds coronaviruses could persist on hard surfaces for up to nine days, but can be killed with the right disinfectant - Suggesting Potential Drug Candidates for Coronavirus,
ChemViews Mag. 2020.
Summarizing the current research and suggesting potential drug candidates for treating patients suffering from the 2019-nCoV acute respiratory disease
- LitCovid
Curated literature hub for tracking up-to-date scientific information about COVID-19 - Many publishers and other entities have signed a joint statement to ensure that COVID-19 research findings and data are shared rapidly and openly

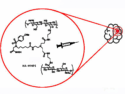

The authors can modify the structure by introducing in molecule of inhibitor the non-natural amino acids, for example homo-phenylalanine instead of phenylalanine or others. Such a structural change often leads to a significant increase in protease inhibition, for example, in the case of inhibition of P. falciparum cysteine proteases. The corresponding molecules were synthesized and showed high inhibition activity. See DOI: 10.1002/cbdv.201700037