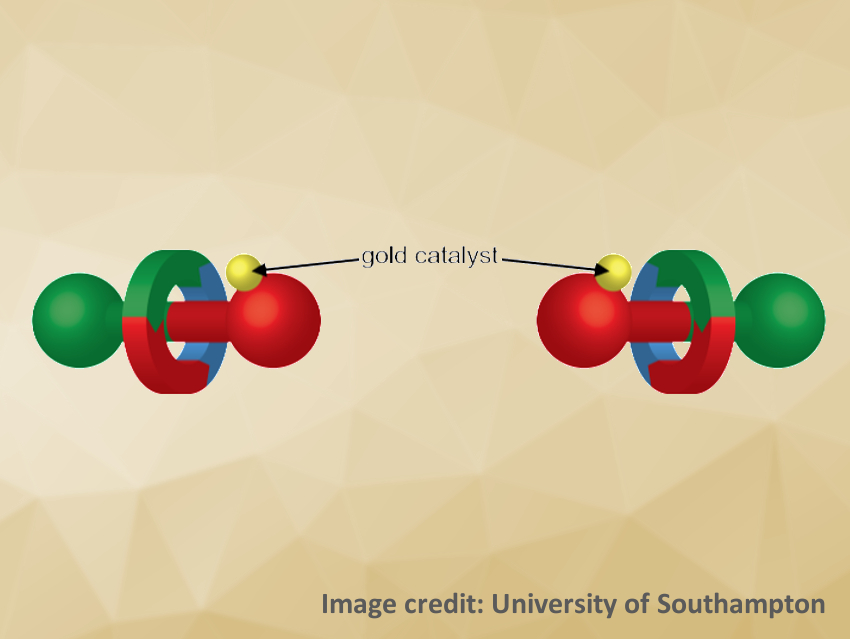
Rotaxanes consist of a ring-shaped molecule wrapped around a dumbbell-shaped “axle” molecule. The ring and axle are held together by a mechanical bond. Chiral rotaxanes have been difficult to synthesize in enantiopure form so far, and their use in asymmetric catalysis has not been well-explored.
Andrew W. Heard and Stephen M. Goldup, University of Southampton, UK, have achieved enantioselective catalysis with a mechanically planar chiral rotaxane for the first time. The rotaxane (schematically pictured) consists of an achiral macrocycle around an achiral axle, which overall creates a chiral structure due to the mechanical bond. This chiral rotaxane can bind gold(I) ions, which were then used to catalyze a cyclopropanation reaction.
The chiral rotaxanes were prepared using a copper-mediated alkyne–azide cycloaddition reaction from an asymmetric azide, an alkyne coupling partner with a phosphine group, and a readily available symmetric macrocycle. The gold(I) was then bound to the phosphine group. The resulting gold complexes were then used to catalyze an enantioselective cyclopropanation of alkenes by propargylic esters.
The team found that the rotaxane-based catalyst achieves stereoselectivities of 42 %–77 % ee in reactions with benzoate esters. This is comparable to optimized conventional, covalent catalysts for the same reaction. This demonstrates that a “mechanically chiral” stereogenic unit can direct enantioselective catalysis.
- Synthesis of a Mechanically Planar Chiral Rotaxane Ligand for Enantioselective Catalysis,
Andrew W. Heard, Stephen M. Goldup,
Chem 2020.
https://doi.org/10.1016/j.chempr.2020.02.006