The chemistry of beryllium has not been extensively investigated. This is due to the element’s high toxicity. Chemical model systems of beryllium with biologically relevant ligands, for example, are rare. This lack of complexes is caused by conflicting solubilities: At physiological pH values, beryllium predominantly forms the insoluble Be(OH)2. Using an alternative solvent could allow more thorough studies of the coordination chemistry of beryllium.
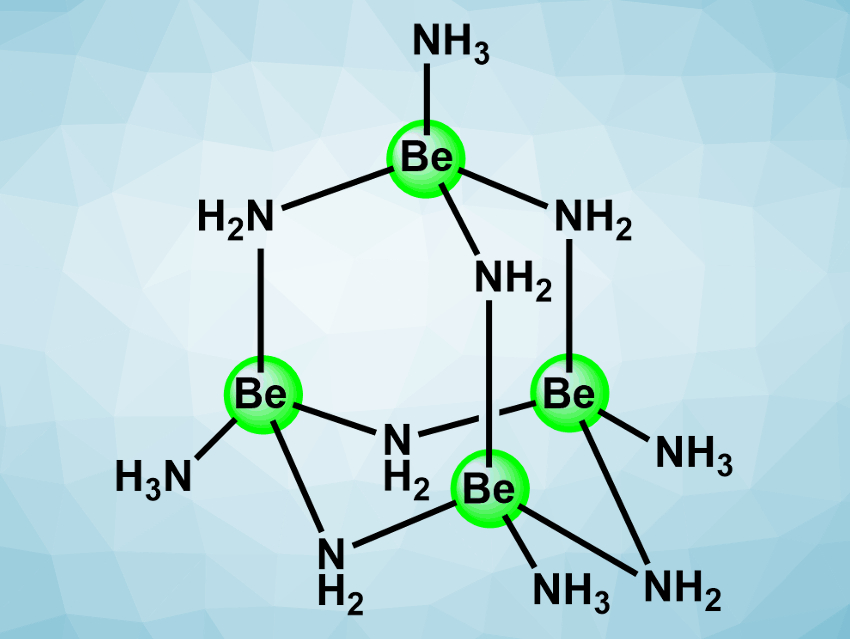
Magnus R. Buchner, University of Marburg, Germany, and colleagues have studied the coordination chemistry of beryllium in liquid ammonia. The team reacted an excess of beryllium metal with the ammonium salts NH4X (X = Cl, Br, I, CN, SCN, N3) in liquid ammonia, which was thawed to room temperature and cooled back down to –78 °C after the reaction. They obtained hexa-µ2-amido-tetraammine-tetraberyllium compounds of the type [Be4(NH2)6(NH3)4]X2 (cation pictured). The products were characterized using X-ray diffraction and infrared (IR,) Raman, and 1H, 9Be, and 15N NMR spectroscopy.
The team found that the tetranuclear beryllium cluster has an adamantyl-like structure (pictured). The team proposes a formation mechanism for this cluster that proceeds via the dimer [Be2(NH2)(NH3)6]3+ and the ringshaped trimer [Be3(NH2)3(NH3)6]3+. The team also obtained [Be8O(NH2)12(py)4]2+, the first example of a cationic beryllium-oxy-amide. This compound was prepared by treating [Be4(NH2)6(NH3)4]I2 with pyridine (py) in the presence of traces of water.
- Speciation of Be2+ in acidic liquid ammonia and formation of tetra- and octanuclear beryllium amido clusters,
Matthias Müller, Antti J. Karttunen, Magnus R. Buchner,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc01112f