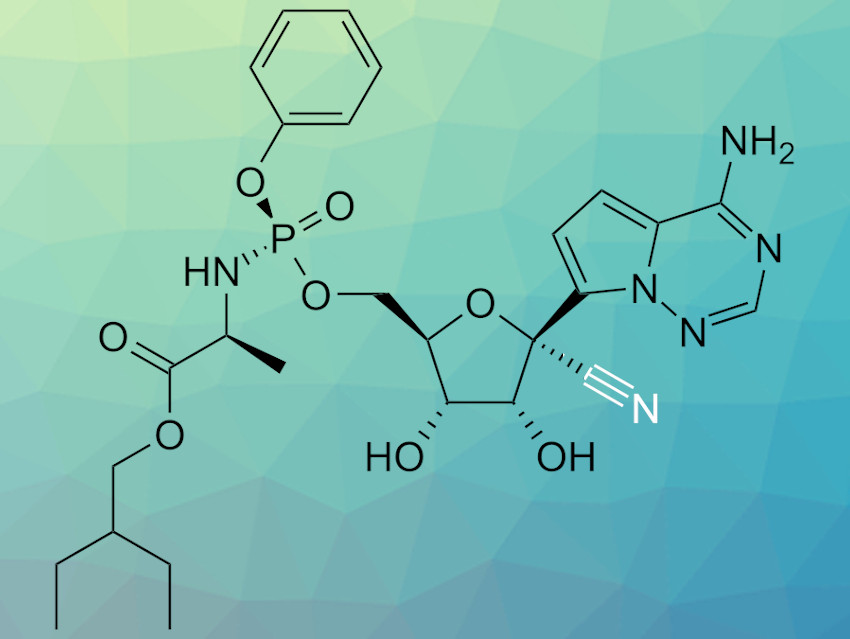
Remdesivir (pictured) is an antiviral compound originally developed to treat Ebola infections. The compound also shows antiviral activity against various coronaviruses and is currently under investigation for the treatment of infections with the coronavirus SARS-CoV-2, responsible for the COVID-19 pandemic. Remdesivir is synthesized via a six-step process that involves a cyanation step. However, this step requires very low temperatures (–78 °C) to achieve the required selectivity and has the potential to produce significant amounts of toxic HCN.
Tiago Vieira, Andrew C. Stevens, Gilead Alberta ULC, Edmonton, Canada, and colleagues have developed a batch synthesis process for the cyanation of a remdesivir precursor that can be operated at –30 °C. Previously, this reaction was performed using trifluoromethanesulfonic acid (TfOH), trimethylsilyl trifluoromethanesulfonate (TMSOTf), and trimethylsilyl cyanide (TMSCN). The team used trifluoroacetic acid (TFA) instead of TfOH and added a precooled mixture of TMSOTf and TMSCN in dichloromethane (DCM) to obtain good diastereoselectivity at –30 °C.
.png)
To allow for the production of larger amounts of the remdesivir precursor, the researchers developed a continuous flow process. In a small-scale lab system, they obtained a yield of 84 % at 99.8 % purity for 100 g of substrate. When the process was tested on a kilogram scale, the team found that the addition of TfOH was required to achieve the same results as in smaller-scale reactions. Using this additional catalyst, the process proved efficient for the large-scale synthesis of the cyanated remdesivir precursor. Only low volumes of dangerous reagents are present in the reactor at any given time, which reduces the risk of HCN exposure. The team obtained 68 kg of the desired product, which allowed the preparation of sufficient amounts of remdesivir for clinical evaluation.
- Development of a Large-Scale Cyanation Process Using Continuous Flow Chemistry En Route to the Synthesis of Remdesivir,
Tiago Vieira, Andrew C. Stevens, Andrei Chtchemelinine, Detian Gao, Pavel Badalov, Lars Heumann,
Org. Process Res. Dev. 2020.
https://doi.org/10.1021/acs.oprd.0c00172
Also of Interest
- Collection: SARS-CoV-2 Virus
What we know about the new coronavirus and COVID-19 - LitCovid
Curated literature hub for tracking up-to-date scientific information about COVID-19 - Many publishers and other entities have signed a joint statement to ensure that COVID-19 research findings and data are shared rapidly and openly