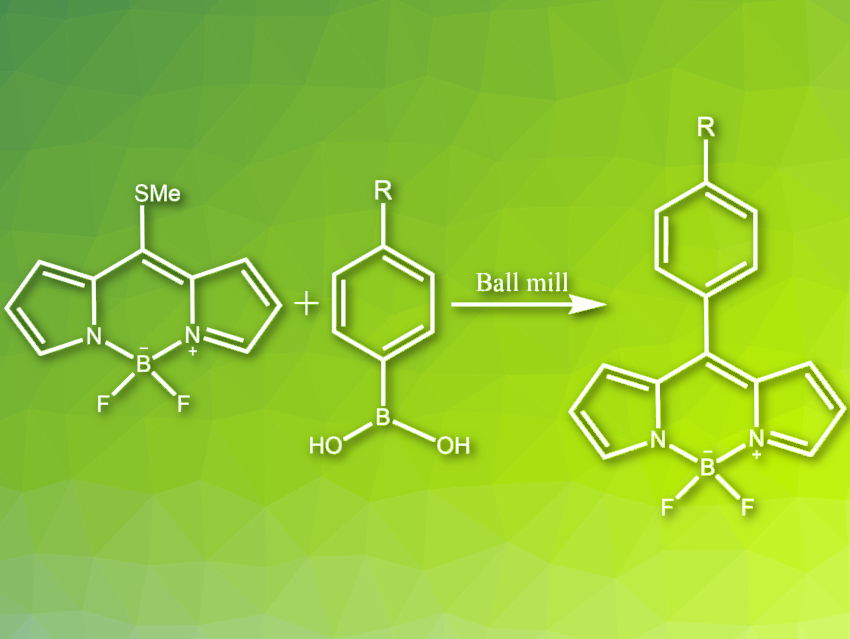
The Liebeskind–Srogl cross-coupling reaction is an efficient method for the synthesis of meso-substituted boron-dipyrromethenes (BODIPYs). This class of fluorescent molecules has many applications, e.g., as photodynamic therapy agents or in organic solar cells. During the Liebeskind–Srogl reaction, carbon–carbon bonds are formed between thioorganics and traditional cross-coupling reagents such as boronic acids or organotin reagents. The reaction uses a Pd(0) catalyst and a copper(I) reagent as an additive. It gives meso-substituted BODIPYs in high yields. However, it requires the strict exclusion of moisture and oxygen, as well as solvents with high boiling points and significant health hazards such as toluene. Therefore, it is not ideal for industrial applications.
Eduardo Peña-Cabrera, University of Guanajuato, Mexico, Eusebio Juaristi, Center for Research and Advanced Studies of the National Polytechnic Institute (CINVESTAV-IPN), Mexico City, Mexico, and colleagues have developed a mechanochemical variant of the Liebeskind–Srogl cross-coupling reaction (pictured). Initially, the reaction was performed in an agate milling jar with an agate ball and a small amount of tetrahydrofuran (THF), using the same reagents as in a tradional approach. After 90 minutes of milling at a frequency of 25 Hz, the results were similar to those obtained under classical reaction conditions, even though air and moisture were not excluded. The reaction was further optimized by using stainless steel instead of agate for the milling jar and ball, reducing the reaction time to 30–45 min. The highest yields were obtained using unsubstituted or electron-deficient aryl boronic acids.
When the process was performed on a larger scale, THF was substituted with ethyl acetate, which is biodegradable, can be produced from biologically produced ethanol and acetic acid, and has considerably lower toxicity than THF or toluene. The products were isolated in yields of 50–95 % by simple crystallization, and the solvent can be re-used after distillation.
- Mechanochemically Activated Liebeskind–Srogl (L-S) Cross-Coupling Reaction: Green Synthesis of meso-Substituted BODIPYs,
Mario Pérez-Venegas, Miriam N. Villanueva-Hernández, Eduardo Peña-Cabrera, Eusebio Juaristi,
Organometallics 2020.
https://doi.org/10.1021/acs.organomet.0c00037