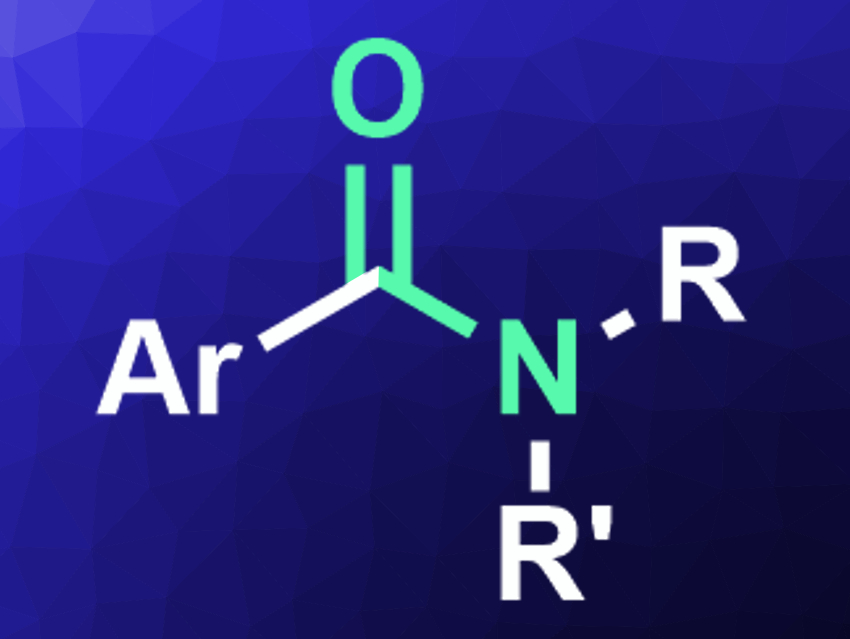
Amides are an important class of compounds found, e.g., in natural products, drugs, or polymers. They are produced in large amounts, usually by reacting carboxylic acid derivatives with amines. However, this pathway requires an additional functionalization step at the carboxylic acid and produces stoichiometric amounts of byproducts. A more efficient, and thus, greener access to amides would be useful. One approach for the development of greener chemical processes is the use of reactions that are mediated by visible light.
Lidia de Luca, University of Sassari, Italy, and colleagues have developed a photocatalyzed amidation reaction of benzyl alcohols with secondary and primary amine hydrochlorides. The team used Ru(bpy)3Cl2 (bpy = bipyridine) as a photosensitizer, tert-butylhydroperoxide (TBHP) as an oxidant, calcium carbonate as a base, and ethyl acetate as the solvent under blue LED light. The reaction was performed at room temperature and the desired amides (pictured) were obtained in good yields.
The reaction tolerates a wide variety of functional groups at the benzyl alcohol, including both electron-donating and electron-withdrawing groups. Due to steric hindrance, only low yields were achieved when benzylic alcohols with bulky ortho-substituents were used. A wide range of aliphatic amine hydrochlorides can be used. However, aromatic amines such as aniline showed no conversion.
The process can be run at room temperature, uses ethyl acetate as a green solvent, and avoids extra steps as well as the generation of halogenated waste. Overall, it provides a green route for the synthesis of amides from stable, inexpensive starting materials.
- Visible-Light Photoredox-Catalyzed Amidation of Benzylic Alcohols,
Silvia Gaspa, Andrea Farina, Mariella Tilocca, Andrea Porcheddu, Luisa Pisano, Massimo Carraro, Ugo Azzena, Lidia De Luca,
J. Org. Chem. 2020.
https://doi.org/10.1021/acs.joc.0c01320