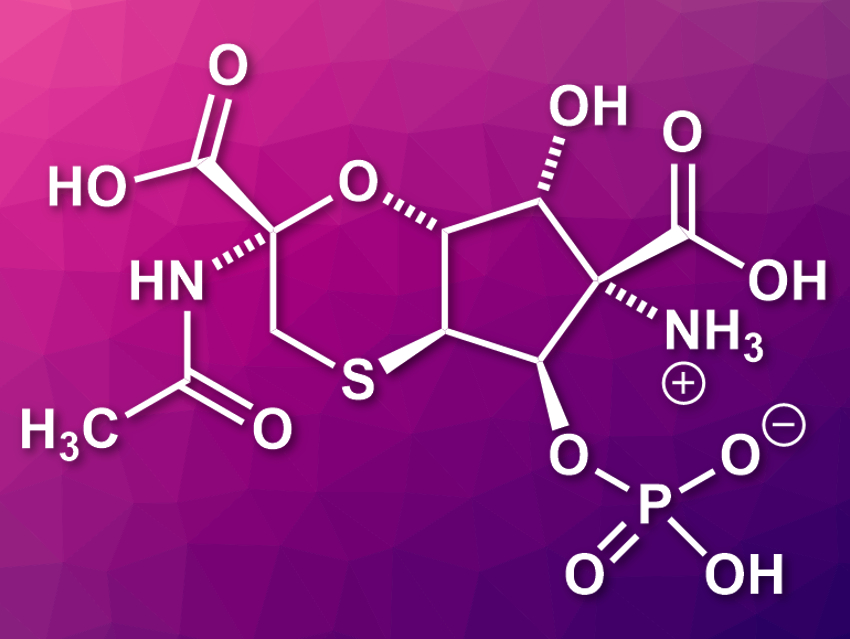
Tagetitoxin (pictured) is a natural product that is produced by Pseudomonas syringae pv. tagetis bacteria. It harms plants by interfering with the development of chloroplasts (the parts of plant cells where photosynthesis is conducted) via the inhibition of RNA polymerase. Tagetitoxin has been a challenging target for structural assignment and total synthesis. Its absolute configuration had not been assigned so far.
Phil S. Baran, Scripps Research, La Jolla, CA, USA, and colleagues have synthesized both enantiomers of tagetitoxin and assigned its absolute configuration. The team started from low-cost, renewable furfural, which was converted to an imine and then subjected to a Mannich addition and an oxidative rearrangement to provide the basis for the fully substituted cyclopentane ring in the product. The resulting intermediate was converted to a thiol via a thio-[3,3] rearrangement to stereoselectively form the C–S bond at the cyclopentane ring. The six-membered ring, a trans-fused 1,4-oxathiane heterocycle, was formed via a bromocyclization using dibromo-5,5-dimethylhydantoin. To install the phosphate group, the team used chiral P(V) reagents, which allowed the team to separate the resulting diastereomers and then obtain both enantiomers of tagetitoxin after a final hydrolysis step.
Overall, the synthesis involves 15 steps and gives both (+)-tagetitoxin and (−)-tagetitoxin. The next step was to assign the absolute configuration of the natural product. However, there is a lack of natural material to compare the products to and no optical rotation data of natural tagetitoxin. Since it is known that the natural product inhibits RNA polymerase, the researchers proposed a configuration assignment based on bioactivity. They used a direct RNA polymerase inhibition assay to probe the bioactivity of each enantiomer and found that only (+)-tagetitoxin showed RNA inhibition. Thus, they conclude that (+)-tagetitoxin must be the correct configuration for the natural product.
- Total Synthesis of Tagetitoxin,
Chi He, Hang Chu, Thomas P. Stratton, David Kossler, Kelly J. Eberle, Dillon T. Flood, Phil S. Baran,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c06641