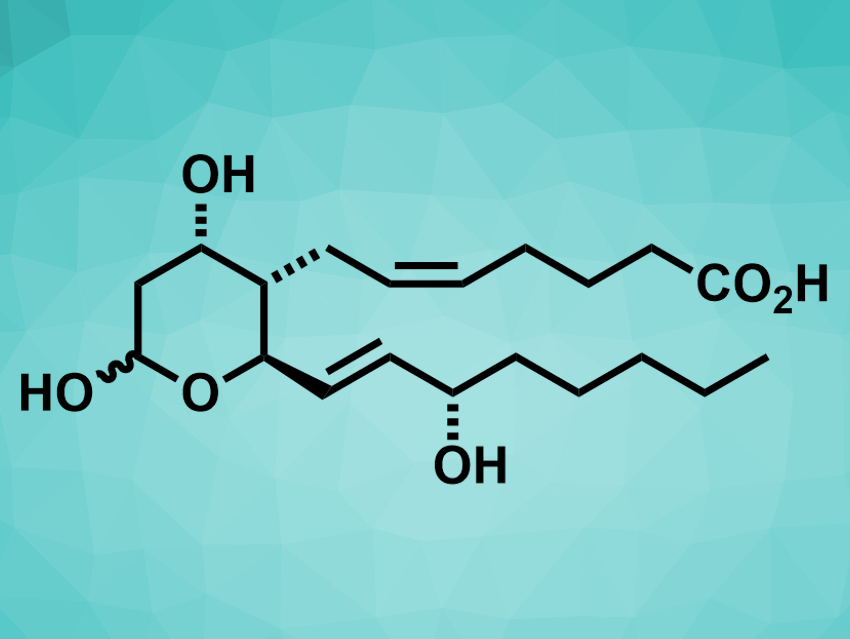
Thromboxane B2 (pictured) is a metabolite of thromboxane A2, a signaling compound that is involved in the formation of blood clots. Thromboxane B2 is useful as a marker and synthetic precursor for thromboxane A2. It also is an interesting target for total synthesis. However, existing approaches to its preparation are lengthy.
Changcheng Jing and Varinder K. Aggarwal, University of Bristol, UK, have developed a concise, twelve-step, asymmetric synthesis of thromboxane B2. The team started from succinaldehyde, which was converted to an enal-lactone using an organocatalytic aldol reaction. The enal-lactone was converted to a para-methoxybenzyl acetal and then subjected to a conjugate addition of an alkenyl side chain, a selective ozonolysis, and a Baeyer–Villiger oxidation to give a key lactone intermediate. A highly selective Wittig olefination was used to install the Z-alkene, followed by deprotection and hydrolysis steps to give the final product.
The researchers obtained thromboxane B2 in twelve steps with an overall yield of 5 %. The enal-lactone intermediate that the team obtained from succinaldehyde can also be used to synthesize other compounds that are structurally related to thromboxane B2.
- Total Synthesis of Thromboxane B2 via a Key Bicyclic Enal Intermediate,
Changcheng Jing, Varinder K. Aggarwal,
Org. Lett. 2020.
https://doi.org/10.1021/acs.orglett.0c02299


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
