Fluorescent compounds have applications, e.g., in organic LEDs (OLEDs). While fluorescent dyes often have useful optical properties, using them to make solid optical materials is challenging. This is due to electronic coupling between them in the solid state that quenches their emission. Decoupling the dye molecules could lead to an entirely new class of bright fluorescent solid materials.
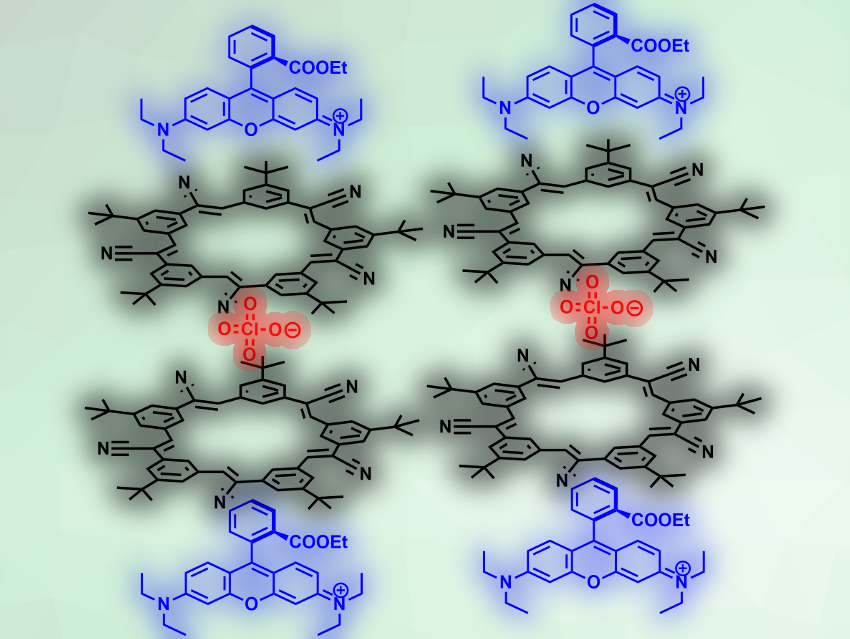
Bo W. Laursen, University of Copenhagen, Denmark, Amar H. Flood, Indiana University, Bloomington, USA, and colleagues have developed such a class of fluorescent materials, which the team calls small-molecule ionic isolation lattices (SMILES). The researchers made the materials by mixing cationic dyes with an ion-binding macrocycle. The dye can, e.g., be rhodamine 3B perchlorate (dye cation pictured in blue, anion pictured in red), which is combined with two equivalents of the anion-binding macrocycle cyanostar (pictured in black).
During crystallization, the macrocycles form a sandwich-type arrangement with the anion. The resulting disc-shaped structures act as spacers between the dye molecules and decouple them electronically in the solid state. The cyanostar–anion complexes are colorless and have wide bandgap, which allows them to efficiently isolate the dye molecules from each other. The team used this principle to prepare fluorescent solids from a wide variety of commercially available cationic dyes. The resulting SMILES-type materials can be blended into different polymers while retaining their emission.
- Plug-and-Play Optical Materials from Fluorescent Dyes and Macrocycles,
Christopher R. Benson, Laura Kacenauskaite, Katherine L. VanDenburgh, Wei Zhao, Bo Qiao, Tumpa Sadhukhan, Maren Pink, Junsheng Chen, Sina Borgi, Chun-Hsing Chen, Brad J. Davis, Yoan C. Simon, Krishnan Raghavachari, Bo W. Laursen, Amar H. Flood,
Chem 2020.
https://doi.org/10.1016/j.chempr.2020.06.029