Covalent organic frameworks (COFs) consist of organic building blocks that form porous, crystalline, two- or three-dimensional networks. Esters are very common in organic chemistry. Ester groups are, for example, used as links in several commonly used polymers, i.e, polyesters such as polyethylene terephthalate (PET). However, preparing ester-linked COFs is challenging because the frameworks need to form orderly, crystalline structures during the esterification reactions.
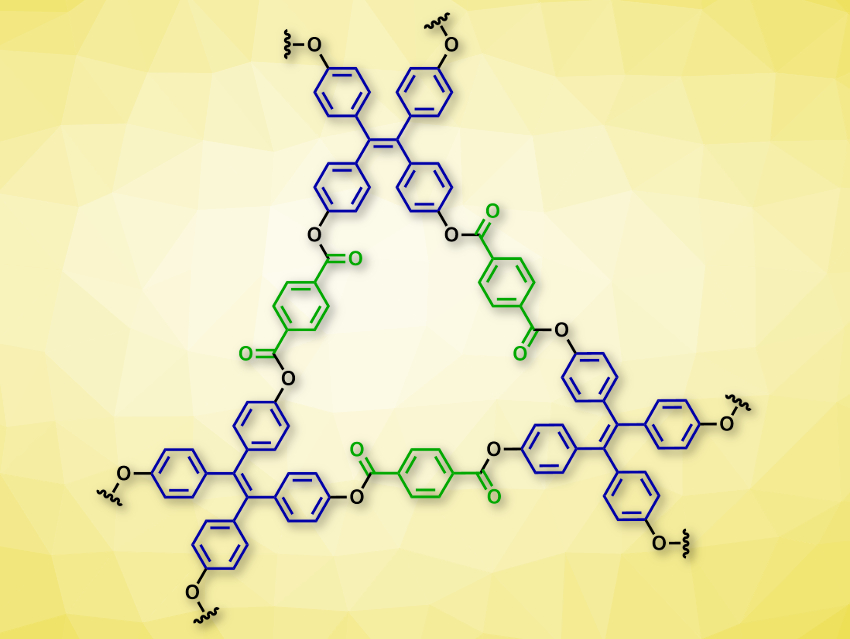
Omar M. Yaghi, University of California, Berkeley, USA, and colleagues have synthesized four different ester-linked, crystalline, porous COFs. The team used terephthalic acid derivatives with two acid sites (example pictured in green) and phenols with three or four hydroxy groups (example pictured in blue) as building blocks. Transesterification reactions then gave the corresponding ester-linked COFs. The researchers used di(pyridin-2-yl) terephthalate (DPT) and tetrakis(4-hydroxyphenyl)ethylene (THPE), which were reacted in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and dioxane at 150 °C, to make the two-dimensional COF-119 (pictured).
The same approach was used to make three other ester-linked COFs: The reaction of 1,3,5-tris(4-hydroxyphenyl)benzene (THPB), which has three hydroxy groups, with DPT gave COF-120. Combining THPB with the larger di-acid building block di(pyridin-2-yl)(1,1′-biphenyl)-4,4′-dicarboxylate (DPBP) gave COF-121. Both form honeycomb-like 2D structures. Using an even larger di-acid building block gave COF-122, the hexagonal COF with the largest edge length known so far. It has a large pore size with a distorted hexagonal structure.
- Ester-Linked Crystalline Covalent Organic Frameworks,
Chenfei Zhao, Hao Lyu, Zhe Ji, Chenhui Zhu, Omar M. Yaghi,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c07015