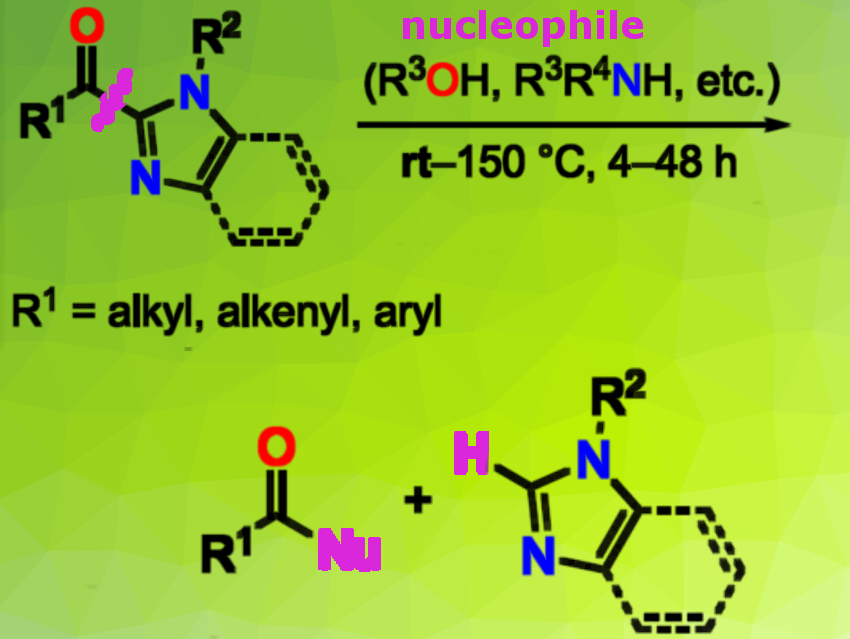
2-Acylimidazoles (pictured left) are widely used as post-transformable carboxylic acid equivalents in chemoselective and enantioselective reactions. Their transformations, however, require pretreatment with highly reactive, toxic methylating reagents such as MeOTf and MeI to facilitate C−C bond cleavage. Such a methylation protocol limits the functional group tolerance, and a protection step is necessary before the transformation to avoid the formation of undesired methylated byproducts.
Hiroyuki Morimoto, Takashi Ohshima, and colleagues, Kyushu University, Fukuoka, Japan, have reported that the C−C bond of unactivated 2-acylimidazoles can be cleaved under neutral conditions without the use of additional reagents or catalysts and without pretreatment. The team transformed the 2-acylimidazoles into the corresponding esters in high yield by heating them in a nucleohile such as alcohol, amine, thiol in a tightly closed reaction vessel to avoid evaporation of the alcohol.
First, the nucleophile attacks the carbonyl moiety of 2-acylimidazole to give a tertiary alcohol intermediate. This intermediate is key to promote the reaction. It is stabilized by the intramolecular hydrogen bonding between the hydroxy group and the imidazole group. Second, the C−C bond is cleaved via a cyclic transition state with simultaneous proton transfer from the tertiary OH group. The C−C bond cleavage proceeds with the assistance of the imidazole moiety.
The team is further investigating the mechanism and if the present protocol can be extended to other transformations.
- C−C Bond Cleavage of Unactivated 2‑Acylimidazoles,
Hai-Long Xin, Bo Pang, Jeesoo Choi, Walaa Akkad, Hiroyuki Morimoto, Takashi Ohshima,
J. Org. Chem. 2020.
https://dx.doi.org/10.1021/acs.joc.0c01458