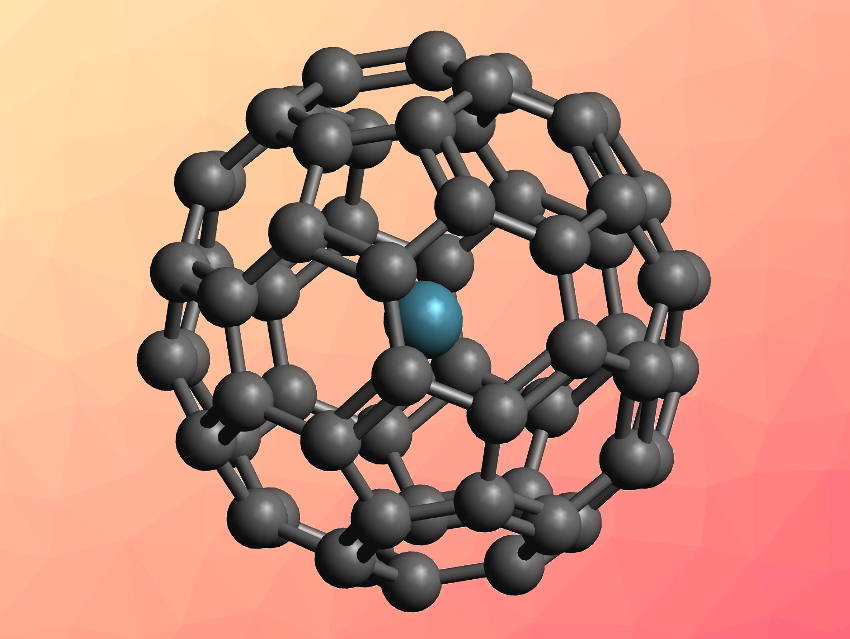
Fullerenes, such as the famous “buckyball” C60, can encapsulate atoms or small molecules to form so-called endohedral fullerenes. Noble gas atoms (He, Ne, Ar, Kr, Xe), for example, can fit into C60. However, synthesizing endohedral fullerenes with the heavier noble gases, such as Ar, is challenging. Direct encapsulation at high temperatures and under high pressures is possible, but gives very low yields. This has limited the amount of Ar@C60 available for study and characterization.
Richard J. Whitby and colleagues, University of Southampton, UK, have developed an improved synthesis of Ar@C60 that is based on filling an open fullerene derivative with argon and then closing the opening via chemical reactions. The team started from an open C60 derivative where the edge is functionalized, among other groups, with a sulfur atom. First, the open cage was filled with an argon atom at 180 °C under ca. 1400 atm of argon. The sulfur unit in the cage was then oxidized to give a sulfoxide and the resulting intermediate was subjected to a photochemical desulfinylation. This reaction made the cage opening smaller. A reduction using triisopropyl phosphite and a cycloaddition using N-phenylmaleimide then completely closed the fullerene and gave the desired product.
The team obtained Ar@C60 in an overall yield of 9.6 % from the open fullerene derivative. Starting from C60, this represents a yield of ca. 2.7 %. The researchers prepared 20 mg of pure Ar@C60, which was enough for the first characterization of the fine structure in a solution 13C NMR spectrum. According to the team, the developed synthesis should allow further studies of the structure and reactivity of Ar@C60.
- Synthesis of Ar@C60 using molecular surgery,
Sally Bloodworth, Gabriela Hoffman, Mark C. Walkey, George R. Bacanu, Julie M. Herniman, Malcolm H. Levitt, Richard J. Whitby,
Chem. Commun. 2020.
https://doi.org/10.1039/d0cc04201c