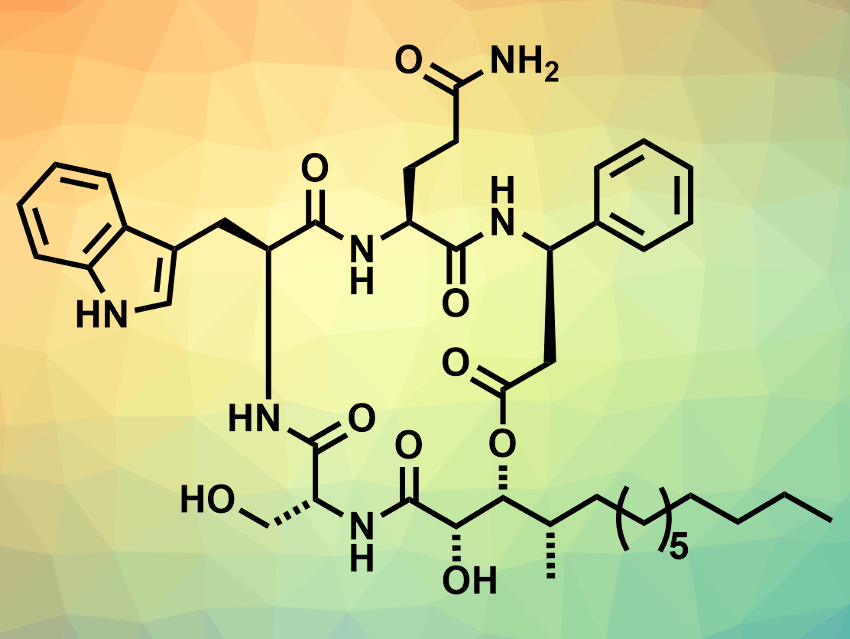
Cyclodepsipeptides are cyclic peptides in which at least one amide group is replaced by the corresponding ester. The cyclodepsipeptides alveolaride A–C were isolated from cultures of the fungus Microascus alveolaris. They are bioactive compounds that might be useful to fight plant pathogens. They are 17-membered macrocycles and contain a rare, non-peptide 2,3-dihydroxy-4-methyltetradecanoic acid (DHMTDA) unit. The structure of alveolaride C (pictured)—in particular, the configuration of the three stereocenters is the DHMTDA unit (shown at the bottom of the picture)—had not been conclusively determined so far.
Rajib Kumar Goswami, School of Chemical Sciences, Indian Association for the Cultivation of Science, Kolkata, and colleagues have performed the first stereoselective total synthesis of alveolaride C and determined the stereochemistry of the three previously unassigned stereocenters. The team used a convergent approach, in which the non-peptide acid unit was subjected to multiple amide couplings and an intermolecular esterification before closing the macrocycle by a lactamization.
The team prepared different enantiomers of the DHMTDA unit, used them in the synthesis of the cyclodepsipeptide, and compared the products’ properties with those of the natural product to assign the configuration of the stereocenters. The NMR and optical rotation data of one of the synthesized stereoisomers matched those of the isolated natural product and was, thus, identified as the target product. Alveolaride C was prepared in 22 linear steps and 6.05 % overall yield.
- Cyclodepsipeptide Alveolaride C: Total Synthesis and Structural Assignment,
Sanu Saha, Debobrata Paul, Rajib Kumar Goswami,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc04478d