Covalent organic frameworks (COFs) are porous crystalline networks made from organic building blocks. They can, for example, be useful in gas adsorption/separation, sensing, or catalysis. However, the synthesis of chiral COF variants (CCOFs) and CCOF-based asymmetric catalysis are not as well developed as the chemistry of achiral COFs. CCOFs can be made via the polymerization of chiral organic monomers, by functionalizing existing COFs with chiral species, or using chiral induction methods during polymerization. Catalytic asymmetric polymerization, however, had not been used so far.
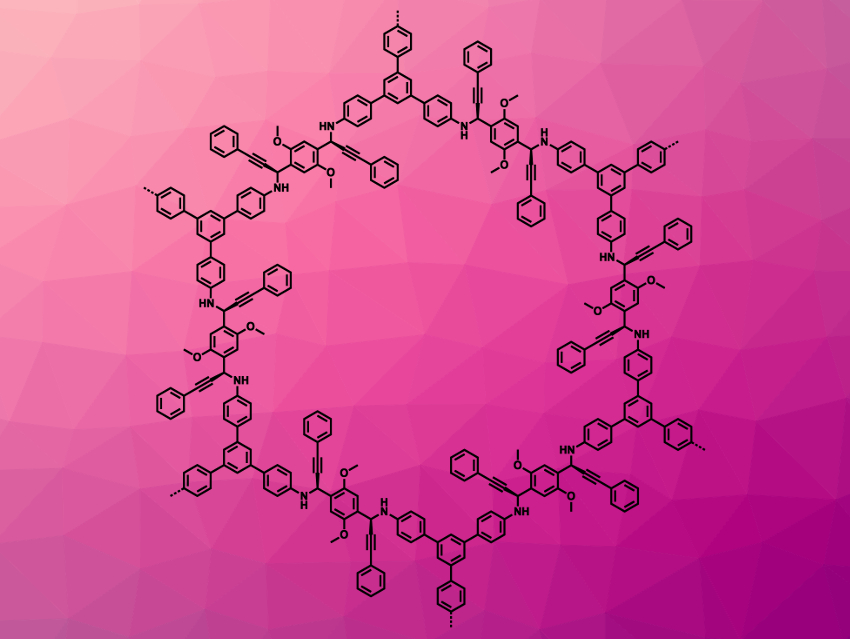
Yu-Bin Dong, Shandong Normal University, Jinan, China, and colleagues have synthesized CCOFs from prochiral monomers via catalytic asymmetric polymerization for the first time. The team prepared the chiral, propargylamine-linked CCOF (S)-DTP-COF (pictured) via an asymmetric polymerization of 2,5-dimethoxyterephthaldehyde, 1,3,5-tris(4-aminophenyl)benzene, and phenylacetylene. They used CuOTf·toluene as a catalyst, together with (S,S)-pybox, a chiral tridentate ligand consisting of a pyridine ring and two oxazoline units. The same reaction with (R,R)-pybox instead of (S,S)-pybox gave the mirrored product (R)-DTP-COF.
The prepared chiral COFs have high optical purities of 93 % ee. They were used as catalysts for asymmetric Michael addition reactions of cyclohexanone with substituted β-nitrostyrenes, giving the desired chiral products in high yields. The CCOFs are highly catalytically active and reusable. According to the researchers, catalytic asymmetric polymerization might be useful to synthesize other new CCOFs.
- Catalytic Asymmetric Synthesis of Chiral Covalent Organic Frameworks from Prochiral Monomers for Heterogeneous Asymmetric Catalysis,
Jian-Cheng Wang, Xuan Kan, Jin-Yan Shang, Hua Qiao, Yu-Bin Dong,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c07461