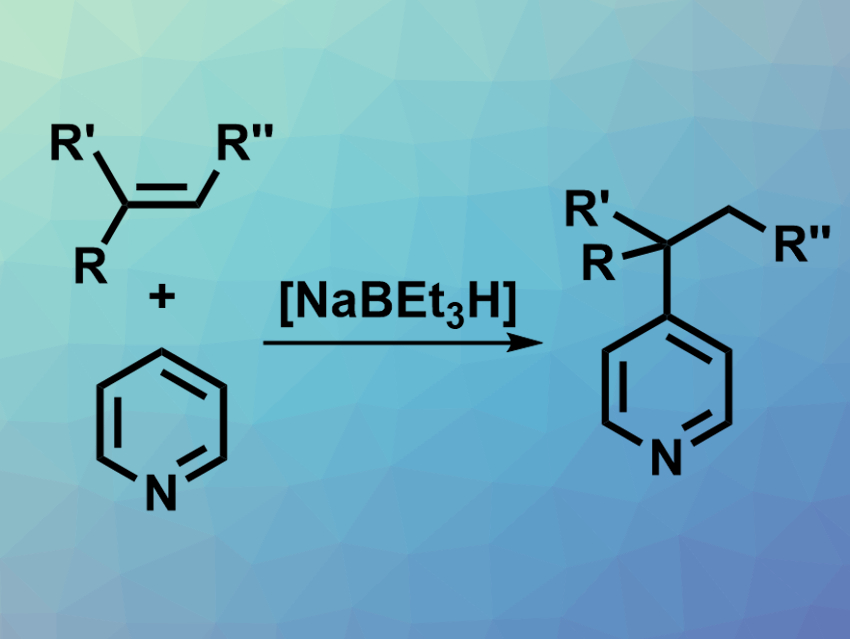
Pyridine derivatives are found in many natural products, pharmaceutically active compounds, functional materials, etc. There is a range of methods for the C2- or C3-functionalization of pyridines, but the introduction of C–C bonds at the C4-position is more challenging. Existing methods rely on transition-metal catalysts, cannot generate all-carbon quaternary centers, and suffer from side reactions.
Tao Xiong, Northeast Normal University, Changchun, China, Qian Zhang, Northeast Normal University and Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and colleagues have developed a NaBEt3H-catalyzed intermolecular Chichibabin-type alkylation of pyridines using alkenes as latent nucleophiles (pictured). The team used various substituted or fused pyridine derivatives as substrates, a variety of styrenes as alkene components, NaBEt3H as the catalyst, BEt3 as an additive, and tetrahydrofuran (THF) as the solvent. The reactions were performed at 100 °C.
The desired alkylated products were obtained in good to excellent yields. The team proposes a reaction mechanism that involves an addition of the hydride catalyst to the alkene, giving an organoborate intermediate. The pyridine is activated by BEt3 and undergoes an addition to the organoborate intermediate. A hydride elimination then gives the desired product and regenerates the catalyst. The reaction is atom-economic, transition-metal-free, and can be used to create triarylmethanes with all-carbon quaternary centers.
- Organoborohydride-Catalyzed Chichibabin-Type C4-Position Alkylation of Pyridines with Alkenes Assisted by Organoborane,
Ying Wang, Run-Han Li, Wei Guan, Yanfei Li, Xiaohong Li, Jianjun Yin, Ge Zhang, Qian Zhang, Tao Xiong, Qian Zhang,
Chem. Sci. 2020.
https://doi.org/10.1039/d0sc04808a