Diamondoids are a class of diamond-like polycyclic compounds, e.g., of the type C4n+6H4n+12. Adamantane is the simplest example of this type of molecule. Due to their structure, these compounds generally have a high thermal stability and show interesting chemical behavior.
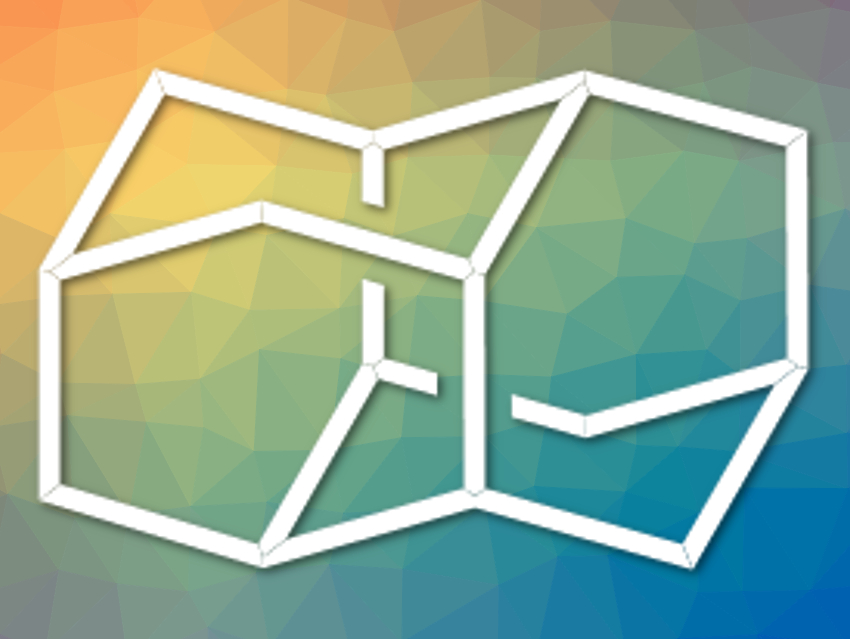
Diamantane, or pentacyclo[7.3.1.14,12.02,7.06,11]tetradecane (pictured), is a diamondoid with the formula С14Н20. It is usually prepared via the isomerization of other strained С14Н20 compounds. These intermediates can be obtained by hydrogenation of the norbornadiene dimer binor-S and isomerize in the presence of superacid catalysts to give diamantane. However, this synthesis of diamantane from binor-S requires harsh hydrogenation conditions and is a two-step process.
Rishat I. Aminov and Ravil I. Khusnutdinov, Institute of Petrochemistry and Catalysis, Russian Academy of Sciences, Ufa, Russia, have developed a direct, one-pot synthesis of diamantane via the hydroisomerization of binor-S in the presence of concentrated sulfuric acid. When this hydroisomerization of binor-S is performed in cyclohexane, it gives a mixture of tetrahydrobinor-S and diamantane. When it is carried out either without a solvent or in CS2, the selectivity for diamantane improves and yields up to 65 % can be achieved.
The team investigated the hydrogen source in the hydroisomerization using deuterated reagents. They found that both sulfuric acid and cyclohexane can serve as the main hydrogen sources. When H2SO4 with a lower concentration of 75–80 % is used, the reaction stops at tetrahydrobinor-S.
- A new method for the synthesis of diamantane by hydroisomerization of binor-S on treatment with sulfuric acid,
Rishat I. Aminov, Ravil I. Khusnutdinov,
Beilstein J. Org. Chem. 2020.
https://doi.org/10.3762/bjoc.16.205


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
